Disease severity, treatment patterns, and quality of life in patients with moderate-to-severe psoriasis routinely managed with systemic treatment: results of the CRYSTAL observational study in Central and Eastern European countries
- PMID: 38846952
- PMCID: PMC11153796
- DOI: 10.3389/fimmu.2024.1410540
Disease severity, treatment patterns, and quality of life in patients with moderate-to-severe psoriasis routinely managed with systemic treatment: results of the CRYSTAL observational study in Central and Eastern European countries
Abstract
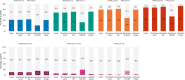
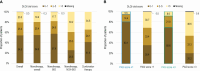
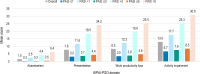
Psoriasis is a common, life-long skin disease with a significant negative health and societal impact. Data on rates of disease control and treatment strategies are lacking in Central and Eastern European countries. We aimed to describe the real-world disease severity, control, and treatment strategies for psoriasis in patients from Central and Eastern European countries. CRYSTAL (EUPAS36459) was a cross-sectional, retrospective study in adults (18-75 years) from Bulgaria, Estonia, Hungary, Latvia, Lithuania, Romania, and Russia. We enrolled patients with moderate-to-severe psoriasis receiving continuous systemic treatment for ≥24 weeks. We used the Psoriasis Area and Severity Index (PASI) to describe disease severity and the Dermatology Life Quality Index (DLQI) to assess quality of life (QoL) and collected other outcomes [psoriasis work productivity and activity impairment (WPAI-PSO), patient satisfaction] at enrollment. Analyses were descriptive. A total of 690 patients were included in the analyses. Median disease duration was 11.8 years. Current treatment was monotherapy for most patients (95.8%) with either biological (BIO group; 88.4%) or conventional (NON-BIO group; 7.4%) agents. Mean (± standard deviation) absolute PASI scores were 3.5 ± 5.7, 3.1 ± 5.3, and 6.6 ± 7.4 in the overall population, the BIO group, and the NON-BIO group, respectively. Among patients treated with monotherapy, absolute PASI scores ≤1, ≤3, and ≤5 were observed for 44.1%, 72.0%, and 82.6% of BIO patients and 21.6%, 33.3%, and 49.0% of NON-BIO patients. Mean DLQI total score was 3.3 ± 5.1; higher scores were noted for higher absolute PASI. The most impacted WPAI-PSO domain was presenteeism; for all domains, impact increased with increased absolute PASI. A total of 91.8% of BIO patients and 74.5% of NON-BIO patients were satisfied with the current treatment. We observed a better disease control in BIO than NON-BIO patients. However, around half of BIO patients did not reach clear skin status and reported an impact on QoL. An improvement in treatment strategies is still needed in Central and Eastern European countries to optimize outcomes of moderate-to-severe psoriasis.
Keywords: patient-reported outcomes; psoriasis; real-world; severity of illness index; systemic therapy.
Copyright © 2024 Raam, Hartmane, Valiukevičienė, Karamova, Telegdy, Botev, Marina, Rubant, Albuquerque and Constantin.
Conflict of interest statement
LR reports honoraria for lectures and support for attending meetings from AbbVie. IH reports grants or contracts from Abbvie and Galderma; consulting fees from AbbVie and Janssen; honoraria for lectures and presentations at educational events from AbbVie, Janssen, and Novartis; and support for attending meetings and/or travel from AbbVie and Janssen. SV reports Investigator agreement and payment from AbbVie. AK reports honoraria for lectures and/or Investigator agreements from AbbVie, Eli Lilly, Pfizer, Regeneron, Janssen, and Leo Pharma. IB reports personal and institution payments from AbbVie. DM, SR, and TA are full-time employees of Abbvie and may hold AbbVie stock and/or stock options. MC reports consulting fees from AbbVie, Novartis Pharma, Eli Lilly, and Pfizer; honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Abbvie, Novartis Pharma, Eli Lilly, Pfizer, Janssen, UCB, Sun Pharma, Genesis Pharma, and Amring; payment for expert testimony from UCB and Amring; and support for attending meetings and/or travel from Terapia and for participation on Data Safety Monitoring or Advisory Board from UCB, Amring, and Novartis Pharma. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from AbbVie. The funder contributed to the design, participated in the collection, analysis, and interpretation of data, and in writing, reviewing, and approval of the final version.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

