Empagliflozin Improves Diastolic Function in HFpEF by Restabilizing the Mitochondrial Respiratory Chain
- PMID: 38847102
- PMCID: PMC11177604
- DOI: 10.1161/CIRCHEARTFAILURE.123.011107
Empagliflozin Improves Diastolic Function in HFpEF by Restabilizing the Mitochondrial Respiratory Chain
Abstract
Background: Clinical studies demonstrated beneficial effects of sodium-glucose-transporter 2 inhibitors on the risk of cardiovascular death in patients with heart failure with preserved ejection fraction (HFpEF). However, underlying processes for cardioprotection remain unclear. The present study focused on the impact of empagliflozin (Empa) on myocardial function in a rat model with established HFpEF and analyzed underlying molecular mechanisms.
Methods: Obese ZSF1 (Zucker fatty and spontaneously hypertensive) rats were randomized to standard care (HFpEF, n=18) or Empa (HFpEF/Empa, n=18). ZSF1 lean rats (con, n=18) served as healthy controls. Echocardiography was performed at baseline and after 4 and 8 weeks, respectively. After 8 weeks of treatment, hemodynamics were measured invasively, mitochondrial function was assessed and myocardial tissue was collected for either molecular and histological analyses or transmission electron microscopy.
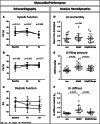
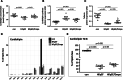
Results: In HFpEF Empa significantly improved diastolic function (E/é: con: 17.5±2.8; HFpEF: 24.4±4.6; P<0.001 versus con; HFpEF/Empa: 19.4±3.2; P<0.001 versus HFpEF). This was accompanied by improved hemodynamics and calcium handling and by reduced inflammation, hypertrophy, and fibrosis. Proteomic analysis demonstrated major changes in proteins involved in mitochondrial oxidative phosphorylation. Cardiac mitochondrial respiration was significantly impaired in HFpEF but restored by Empa (Vmax complex IV: con: 0.18±0.07 mmol O2/s/mg; HFpEF: 0.13±0.05 mmol O2/s/mg; P<0.041 versus con; HFpEF/Empa: 0.21±0.05 mmol O2/s/mg; P=0.012 versus HFpEF) without alterations of mitochondrial content. The expression of cardiolipin, an essential stability/functionality-mediating phospholipid of the respiratory chain, was significantly decreased in HFpEF but reverted by Empa (con: 15.9±1.7 nmol/mg protein; HFpEF: 12.5±1.8 nmol/mg protein; P=0.002 versus con; HFpEF/Empa: 14.5±1.8 nmol/mg protein; P=0.03 versus HFpEF). Transmission electron microscopy revealed a reduced size of mitochondria in HFpEF, which was restored by Empa.
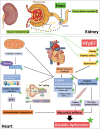
Conclusions: The study demonstrates beneficial effects of Empa on diastolic function, hemodynamics, inflammation, and cardiac remodeling in a rat model of HFpEF. These effects were mediated by improved mitochondrial respiratory capacity due to modulated cardiolipin and improved calcium handling.
Keywords: cardiolipins; empagliflozin; heart failure; hypertension; inflammation.
Conflict of interest statement
Figures




Comment in
-
Proving SGLT2 Inhibitor-Mediated Improvement in Cardiomyocyte Energetics: Beyond a Reasonable Doubt?Circ Heart Fail. 2024 Jun;17(6):e011646. doi: 10.1161/CIRCHEARTFAILURE.124.011646. Epub 2024 Jun 7. Circ Heart Fail. 2024. PMID: 38847114 Free PMC article. No abstract available.
References
-
- Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530 - PubMed
-
- Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–573. doi: 10.1038/s41569-020-0363-2 - PubMed
-
- Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, et al. . Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation. 2021;144:1284–1294. doi: 10.1161/CIRCULATIONAHA.121.056824 - PMC - PubMed
-
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. ; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

