Microbial metabolites affect tumor progression, immunity and therapy prediction by reshaping the tumor microenvironment (Review)
- PMID: 38847233
- PMCID: PMC11173369
- DOI: 10.3892/ijo.2024.5661
Microbial metabolites affect tumor progression, immunity and therapy prediction by reshaping the tumor microenvironment (Review)
Abstract
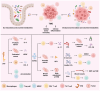
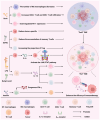
Several studies have indicated that the gut microbiome and tumor microbiota may affect tumors. Emerging metabolomics research illustrates the need to examine the variations in microbial metabolite composition between patients with cancer and healthy individuals. Microbial metabolites can impact the progression of tumors and the immune response by influencing a number of mechanisms, including modulation of the immune system, cancer or immune‑related signaling pathways, epigenetic modification of proteins and DNA damage. Microbial metabolites can also alleviate side effects and drug resistance during chemotherapy and immunotherapy, while effectively activating the immune system to exert tumor immunotherapy. Nevertheless, the impact of microbial metabolites on tumor immunity can be both beneficial and harmful, potentially influenced by the concentration of the metabolites or the specific cancer type. The present review summarizes the roles of various microbial metabolites in different solid tumors, alongside their influence on tumor immunity and treatment. Additionally, clinical trials evaluating the therapeutic effects of microbial metabolites or related microbes on patients with cancer have been listed. In summary, studying microbial metabolites, which play a crucial role in the interaction between the microbiota and tumors, could lead to the identification of new supplementary treatments for cancer. This has the potential to improve the effectiveness of cancer treatment and enhance patient prognosis.
Keywords: cancer; chemotherapy; gut microbiome; immunotherapy; microbial metabolites; tumor microbiota; tumor microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Metabolic mediators: microbial-derived metabolites as key regulators of anti-tumor immunity, immunotherapy, and chemotherapy.Front Immunol. 2024 Sep 16;15:1456030. doi: 10.3389/fimmu.2024.1456030. eCollection 2024. Front Immunol. 2024. PMID: 39351241 Free PMC article. Review.
-
Gut microbial metabolites in cancer therapy.Trends Endocrinol Metab. 2025 Jan;36(1):55-69. doi: 10.1016/j.tem.2024.06.016. Epub 2024 Jul 14. Trends Endocrinol Metab. 2025. PMID: 39004537 Review.
-
The gut microbiome modulate response to immunotherapy in cancer.Sci China Life Sci. 2025 Feb;68(2):381-396. doi: 10.1007/s11427-023-2634-7. Epub 2024 Sep 2. Sci China Life Sci. 2025. PMID: 39235561 Review.
-
The Role of Gut Microbiota in Tumor Immunotherapy.J Immunol Res. 2021 Aug 26;2021:5061570. doi: 10.1155/2021/5061570. eCollection 2021. J Immunol Res. 2021. PMID: 34485534 Free PMC article. Review.
-
An emerging strategy: probiotics enhance the effectiveness of tumor immunotherapy via mediating the gut microbiome.Gut Microbes. 2024 Jan-Dec;16(1):2341717. doi: 10.1080/19490976.2024.2341717. Epub 2024 May 8. Gut Microbes. 2024. PMID: 38717360 Free PMC article. Review.
Cited by
-
Revealing gut microbiota biomarkers associated with melanoma immunotherapy response and key bacteria-fungi interaction relationships: evidence from metagenomics, machine learning, and SHAP methodology.Front Immunol. 2025 Mar 18;16:1539653. doi: 10.3389/fimmu.2025.1539653. eCollection 2025. Front Immunol. 2025. PMID: 40170844 Free PMC article.
-
Dissecting the Genetic Correlations and Causal Effects of Gut Microbiota on Pan-Cancer Phenotype: Driven by Common Dietary Preferences.Food Sci Nutr. 2025 Jul 28;13(8):e70708. doi: 10.1002/fsn3.70708. eCollection 2025 Aug. Food Sci Nutr. 2025. PMID: 40735410 Free PMC article.
-
The role of the gut microbiota in shaping the tumor microenvironment and immunotherapy of breast cancer.Front Microbiol. 2025 May 27;16:1591745. doi: 10.3389/fmicb.2025.1591745. eCollection 2025. Front Microbiol. 2025. PMID: 40497049 Free PMC article. Review.
-
Effect of Nematodes-Bacteria Complex Metabolites on Cancer and Tumor Progression.Biomolecules. 2025 Aug 14;15(8):1165. doi: 10.3390/biom15081165. Biomolecules. 2025. PMID: 40867609 Free PMC article. Review.
-
Cancer and the Microbiome of the Human Body.Nutrients. 2024 Aug 21;16(16):2790. doi: 10.3390/nu16162790. Nutrients. 2024. PMID: 39203926 Free PMC article. Review.
References
-
- Damelin M, Bankovich A, Bernstein J, Lucas J, Chen L, Williams S, Park A, Aguilar J, Ernstoff E, Charati M, et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med. 2017;9:eaag2611. doi: 10.1126/scitranslmed.aag2611. - DOI - PubMed
-
- Srivastava S, Furlan SN, Jaeger-Ruckstuhl CA, Sarvothama M, Berger C, Smythe KS, Garrison SM, Specht JM, Lee SM, Amezquita RA, et al. Immunogenic chemotherapy enhances recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell. 2021;39:193–208.e10. doi: 10.1016/j.ccell.2020.11.005. - DOI - PMC - PubMed
-
- Zhao Y, Li ZX, Zhu YJ, Fu J, Zhao XF, Zhang YN, Wang S, Wu JM, Wang KT, Wu R, et al. Single-Cell transcriptome analysis uncovers intratumoral heterogeneity and underlying mechanisms for drug resistance in hepatobiliary tumor organoids. Adv Sci (Weinh) 2021;8:e2003897. doi: 10.1002/advs.202003897. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
