Glutathione‑degrading enzymes in the complex landscape of tumors (Review)
- PMID: 38847236
- PMCID: PMC11173371
- DOI: 10.3892/ijo.2024.5660
Glutathione‑degrading enzymes in the complex landscape of tumors (Review)
Abstract
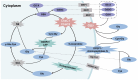
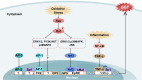
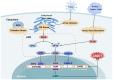
Glutathione (GSH)‑degrading enzymes are essential for starting the first stages of GSH degradation. These enzymes include extracellular γ‑glutamyl transpeptidase (GGT) and intracellular GSH‑specific γ‑glutamylcyclotransferase 1 (ChaC1) and 2. These enzymes are essential for cellular activities, such as immune response, differentiation, proliferation, homeostasis regulation and programmed cell death. Tumor tissue frequently exhibits abnormal expression of GSH‑degrading enzymes, which has a key impact on the development and spread of malignancies. The present review summarizes gene and protein structure, catalytic activity and regulation of GSH‑degrading enzymes, their vital roles in tumor development (including regulation of oxidative and endoplasmic reticulum stress, control of programmed cell death, promotion of inflammation and tumorigenesis and modulation of drug resistance in tumor cells) and potential role as diagnostic biomarkers and therapeutic targets.
Keywords: ChaC1; GGT; GSH degrading enzyme; tumor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
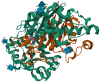

Similar articles
-
Signaling pathways upregulating glutathione‑specific γ‑glutamylcyclotransferase 1 by 3‑(5'‑hydroxymethyl‑2'‑furyl)‑1‑benzylindazole.Mol Med Rep. 2023 Nov;28(5):216. doi: 10.3892/mmr.2023.13103. Epub 2023 Sep 29. Mol Med Rep. 2023. PMID: 37772365
-
Differential regulation of γ-glutamyltransferase and glutamate cysteine ligase expression after mitochondrial uncoupling: γ-glutamyltransferase is regulated in an Nrf2- and NFκB-independent manner.Free Radic Res. 2013 May;47(5):394-403. doi: 10.3109/10715762.2013.781270. Epub 2013 Apr 8. Free Radic Res. 2013. PMID: 23448276
-
Heart failure and the glutathione cycle: an integrated view.Biochem J. 2020 Sep 18;477(17):3123-3130. doi: 10.1042/BCJ20200429. Biochem J. 2020. PMID: 32886767 Review.
-
Gamma-Glutamylcyclotransferase: A Novel Target Molecule for Cancer Diagnosis and Treatment.Biomed Res Int. 2015;2015:345219. doi: 10.1155/2015/345219. Epub 2015 Aug 3. Biomed Res Int. 2015. PMID: 26339607 Free PMC article. Review.
-
Gamma-glutamyl transpeptidase: redox regulation and drug resistance.Adv Cancer Res. 2014;122:103-41. doi: 10.1016/B978-0-12-420117-0.00003-7. Adv Cancer Res. 2014. PMID: 24974180 Free PMC article. Review.
Cited by
-
Initial Glutathione Depletion During Short-Term Bed Rest: Pinpointing Synthesis and Degradation Checkpoints in the γ-Glutamyl Cycle.Antioxidants (Basel). 2024 Nov 21;13(12):1430. doi: 10.3390/antiox13121430. Antioxidants (Basel). 2024. PMID: 39765759 Free PMC article.
-
Role of exosomal non‑coding RNAs in cancer‑associated fibroblast‑mediated therapy resistance (Review).Int J Oncol. 2025 Aug;67(2):68. doi: 10.3892/ijo.2025.5774. Epub 2025 Jul 19. Int J Oncol. 2025. PMID: 40682844 Free PMC article. Review.
-
Molecular mechanisms and therapeutic strategies in overcoming chemotherapy resistance in cancer.Mol Biomed. 2025 Jan 6;6(1):2. doi: 10.1186/s43556-024-00239-2. Mol Biomed. 2025. PMID: 39757310 Free PMC article. Review.
-
A Review Unveiling the Ferroptosis-Regulated Cell Signalling Pathways in Breast Cancer to Elucidate Potent Targets for Cancer Management.Curr Pharm Des. 2025;31(20):1593-1603. doi: 10.2174/0113816128343266241230045019. Curr Pharm Des. 2025. PMID: 39901686 Review.
-
Prognostic effect of pretreatment serum gamma-glutamyl transferase in urological malignancies: a systematic review and meta-analysis.Front Oncol. 2025 Jun 25;15:1597155. doi: 10.3389/fonc.2025.1597155. eCollection 2025. Front Oncol. 2025. PMID: 40636684 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
