Exploring of N-phthalimide-linked 1,2,3-triazole analogues with promising -anti-SARS-CoV-2 activity: synthesis, biological screening, and molecular modelling studies
- PMID: 38847308
- PMCID: PMC11164105
- DOI: 10.1080/14756366.2024.2351861
Exploring of N-phthalimide-linked 1,2,3-triazole analogues with promising -anti-SARS-CoV-2 activity: synthesis, biological screening, and molecular modelling studies
Abstract
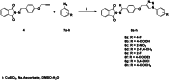
In this study, a library of phthalimide Schiff base linked to 1,4-disubstituted-1,2,3-triazoles was designed, synthesised, and characterised by different spectral analyses. All analogues have been introduced for in vitro assay of their antiviral activity against COVID-19 virus using Vero cell as incubator with different concentrations. The data revealed most of these derivatives showed potent cellular anti-COVID-19 activity and prevent viral growth by more than 90% at two different concentrations with no or weak cytotoxic effect on Vero cells. Furthermore, in vitro assay was done against this enzyme for all analogues and the results showed two of them have IC50 data by 90 µM inhibitory activity. An extensive molecular docking simulation was run to analyse their antiviral mechanism that found the proper non-covalent interaction within the Mpro protease enzyme. Finally, we profiled two reversible inhibitors, COOH and F substituted analogues that might be promising drug candidates for further development have been discovered.
Keywords: 123-Triazole; COVID-19; Schiff bases; molecular docking; phtalamide.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




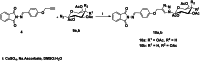


Similar articles
-
Chloropyridinyl Esters of Nonsteroidal Anti-Inflammatory Agents and Related Derivatives as Potent SARS-CoV-2 3CL Protease Inhibitors.Molecules. 2021 Sep 24;26(19):5782. doi: 10.3390/molecules26195782. Molecules. 2021. PMID: 34641337 Free PMC article.
-
Discovery and Mechanism of SARS-CoV-2 Main Protease Inhibitors.J Med Chem. 2022 Feb 24;65(4):2866-2879. doi: 10.1021/acs.jmedchem.1c00566. Epub 2021 Sep 27. J Med Chem. 2022. PMID: 34570513 Free PMC article.
-
Design, synthesis, and biological activity evaluation of dihydromyricetin derivatives against SARS-CoV-2-Omicron virus.J Enzyme Inhib Med Chem. 2024 Dec;39(1):2390909. doi: 10.1080/14756366.2024.2390909. Epub 2024 Aug 29. J Enzyme Inhib Med Chem. 2024. PMID: 39206852 Free PMC article.
-
Protease targeted COVID-19 drug discovery: What we have learned from the past SARS-CoV inhibitors?Eur J Med Chem. 2021 Apr 5;215:113294. doi: 10.1016/j.ejmech.2021.113294. Epub 2021 Feb 13. Eur J Med Chem. 2021. PMID: 33618158 Free PMC article. Review.
-
Repurposing an Antiviral Drug against SARS-CoV-2 Main Protease.Angew Chem Int Ed Engl. 2021 Oct 25;60(44):23492-23494. doi: 10.1002/anie.202107481. Epub 2021 Sep 21. Angew Chem Int Ed Engl. 2021. PMID: 34545983 Free PMC article. Review.
Cited by
-
Insights into SARS-CoV-2: Small-Molecule Hybrids for COVID-19 Treatment.Molecules. 2024 Nov 15;29(22):5403. doi: 10.3390/molecules29225403. Molecules. 2024. PMID: 39598790 Free PMC article. Review.
References
-
- Lengerli D, Ibis K, Nural Y, Banoglu E.. The 1, 2, 3-triazole ‘all-in-one’ring system in drug discovery: A good bioisostere, a good pharmacophore, a good linker, and a versatile synthetic tool. Expert Opin Drug Discov. 2022;17(11):1209–1236. - PubMed
-
- Hashimoto Y. Thalidomide as a multi-template for development of biologically active compounds. Arch Pharm (Weinheim)). 2008;341(9):536–547. - PubMed
-
- Shinji C, Nakamura T, Maeda S, Yoshida M, Hashimoto Y, Miyachi H.. Design and synthesis of phthalimide-type histone deacetylase inhibitors. Bioorg Med Chem Lett. 2005;15(20):4427–4431. - PubMed
-
- Wang F, Yin H, Yue C, Cheng S, Hong M.. Syntheses, structural characterization, in vitro cytotoxicities and DNA-binding properties of triphenylantimony di (N-oxy phthalimide) and di (N-oxy succinimide) complexes. J Organomet Chem. 2013;738:35–40.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
