MALAT1/miR-7-5p/TCF4 Axis Regulating Menstrual Blood Mesenchymal Stem Cells Improve Thin Endometrium Fertility by the Wnt Signaling Pathway
- PMID: 38847385
- PMCID: PMC11162126
- DOI: 10.1177/09636897241259552
MALAT1/miR-7-5p/TCF4 Axis Regulating Menstrual Blood Mesenchymal Stem Cells Improve Thin Endometrium Fertility by the Wnt Signaling Pathway
Abstract
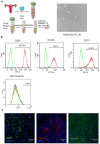
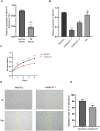
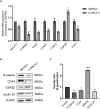
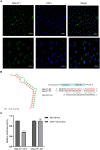
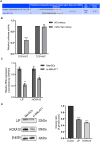
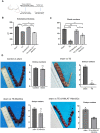
Thin endometrium (TE) is a significant factor contributing to fertility challenges, and addressing this condition remains a central challenge in reproductive medicine. Menstrual blood-derived mesenchymal stem cells (MenSCs) play a crucial role in tissue repair and regeneration, including that of TE. The Wnt signaling pathway, which is highly conserved and prevalent in eukaryotes, is essential for cell proliferation, tissue development, and reproductive functions. MALAT1 is implicated in various transcriptional and molecular functions, including cell proliferation and metastasis. However, the combined effects of the Wnt signaling pathway and the long non-coding RNA (lncRNA) MALAT1 on the regulation of MenSCs' regenerative capabilities in tissue engineering have not yet been explored. To elucidate the regulatory mechanism of MALAT1 in TE, we analyzed its expression levels in normal endometrium and TE tissues, finding that low expression of MALAT1 was associated with poor clinical prognosis. In addition, we conducted both in vitro and in vivo functional assays to examine the role of the MALAT1/miR-7-5p/TCF4 axis in cell proliferation and migration. Techniques such as dual-luciferase reporter assay, fluorescent in situ hybridization, and immunoblot experiments were utilized to clarify the molecular mechanism. To corroborate these findings, we established a TE model and conducted pregnancy experiments, demonstrating a strong association between MALAT1 expression and endometrial fertility. In conclusion, our comprehensive study provides strong evidence supporting that lncRNA MALAT1 modulates TCF4 expression in the Wnt signaling pathway through interaction with miR-7-5p, thus enhancing MenSCs-mediated improvement of TE and improving fertility.
Keywords: Wnt signaling pathway; endometrial fertility; long non-coding RNA; menstrual blood–derived mesenchymal stem cells; thin endometrium.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Investigations on the mechanism of progesterone in inhibiting endometrial cancer cell cycle and viability via regulation of long noncoding RNA NEAT1/microRNA-146b-5p mediated Wnt/β-catenin signaling.IUBMB Life. 2019 Feb;71(2):223-234. doi: 10.1002/iub.1959. Epub 2018 Nov 19. IUBMB Life. 2019. PMID: 30452118
-
LncRNA MALAT1/miR-181a-5p affects the proliferation and adhesion of myeloma cells via regulation of Hippo-YAP signaling pathway.Cell Cycle. 2019 Oct;18(19):2509-2523. doi: 10.1080/15384101.2019.1652034. Epub 2019 Aug 9. Cell Cycle. 2019. PMID: 31397203 Free PMC article.
-
miR-425-5p decreases LncRNA MALAT1 and TUG1 expressions and suppresses tumorigenesis in osteosarcoma via Wnt/β-catenin signaling pathway.Int J Biochem Cell Biol. 2019 Jun;111:42-51. doi: 10.1016/j.biocel.2019.04.004. Epub 2019 Apr 12. Int J Biochem Cell Biol. 2019. PMID: 30986552
-
Long non-coding RNA MALAT1 as a key target in pathogenesis of glioblastoma. Janus faces or Achilles' heal?Gene. 2020 May 20;739:144518. doi: 10.1016/j.gene.2020.144518. Epub 2020 Feb 28. Gene. 2020. PMID: 32119915 Review.
-
Steps towards the clinical application of endometrial and menstrual fluid mesenchymal stem cells for the treatment of gynecological disorders.Expert Opin Biol Ther. 2025 Mar;25(3):285-307. doi: 10.1080/14712598.2025.2465826. Epub 2025 Feb 19. Expert Opin Biol Ther. 2025. PMID: 39925343 Review.
Cited by
-
Long-term therapeutic effects of allogeneic mesenchymal stem cell transplantation for intrauterine adhesions.Stem Cell Res Ther. 2024 Dec 23;15(1):499. doi: 10.1186/s13287-024-04100-9. Stem Cell Res Ther. 2024. PMID: 39716301 Free PMC article.
References
-
- Yao Q, Zheng YW, Lan QH, Wang LF, Huang ZW, Chen R, Yang Y, Xu HL, Kou L, Zhao YZ. Aloe/poloxamer hydrogel as an injectable beta-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment. Eur J Pharm Sci. 2020;148:105316. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

