Enhancing CRISPR prime editing by reducing misfolded pegRNA interactions
- PMID: 38847802
- PMCID: PMC11161173
- DOI: 10.7554/eLife.90948
Enhancing CRISPR prime editing by reducing misfolded pegRNA interactions
Abstract
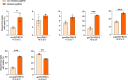
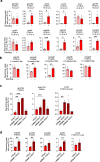
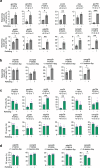
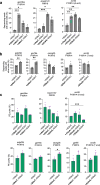
CRISPR prime editing (PE) requires a Cas9 nickase-reverse transcriptase fusion protein (known as PE2) and a prime editing guide RNA (pegRNA), an extended version of a standard guide RNA (gRNA) that both specifies the intended target genomic sequence and encodes the desired genetic edit. Here, we show that sequence complementarity between the 5' and the 3' regions of a pegRNA can negatively impact its ability to complex with Cas9, thereby potentially reducing PE efficiency. We demonstrate this limitation can be overcome by a simple pegRNA refolding procedure, which improved ribonucleoprotein-mediated PE efficiencies in zebrafish embryos by up to nearly 25-fold. Further gains in PE efficiencies of as much as sixfold could also be achieved by introducing point mutations designed to disrupt internal interactions within the pegRNA. Our work defines simple strategies that can be implemented to improve the efficiency of PE.
Keywords: RNA structure; gene editing; genetics; genomics; protein delivery; zebrafish.
© 2023, Zhang et al.
Conflict of interest statement
WZ, JM, HL, CT, JY No competing interests declared, KP has a financial interest in SeQure Dx, Inc. KP's interests and relationships have been disclosed to Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies, JJ has, or had during the course of this research, financial interests in several companies developing gene editing technology: Beam Therapeutics, Blink Therapeutics, Chroma Medicine, Editas Medicine, EpiLogic Therapeutics, Excelsior Genomics, Hera Biolabs, Monitor Biotechnologies, Nvelop Therapeutics (f/k/a ETx, Inc), Pairwise Plants, Poseida Therapeutics, SeQure Dx, Inc, and Verve Therapeutics. JKJ's interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. JKJ is a co-inventor on various patents and patent applications that describe gene editing and epigenetic editing technologies
Figures

Update of
-
Enhancing CRISPR prime editing by reducing misfolded pegRNA interactions.bioRxiv [Preprint]. 2023 Aug 15:2023.08.14.553324. doi: 10.1101/2023.08.14.553324. bioRxiv. 2023. Update in: Elife. 2024 Jun 07;12:RP90948. doi: 10.7554/eLife.90948. PMID: 37645936 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

