LncRNA MIR181A2HG inhibits keratinocytes proliferation through miR-223-3p/SOX6 axis
- PMID: 38848163
- PMCID: PMC11210253
- DOI: 10.18632/aging.205902
LncRNA MIR181A2HG inhibits keratinocytes proliferation through miR-223-3p/SOX6 axis
Abstract
Background: Psoriasis is a complex and recurrent chronic inflammatory skin disease, and the abnormal proliferation of keratinocytes plays a crucial role in the pathogenesis of psoriasis. Long non-coding RNAs (lncRNAs) play an indispensable role in regulating cellular functions. This research aims to explore the potential impact of lncRNA MIR181A2HG on the regulation of keratinocyte proliferation.
Methods: The expression level of MIR181A2HG and the mRNA level of KRT6, KRT16, and SOX6 were assessed using qRT-PCR. The viability and proliferation of keratinocytes were evaluated using CCK-8 and EdU assays. Cell cycle analysis was performed using flow cytometry. Dual-luciferase reporter assays were applied to test the interaction among MIR181A2HG/miR-223-3p/SOX6. Protein level was detected by Western blotting analysis.
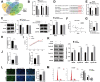
Results: The findings indicated that psoriasis lesions tissue exhibited lower levels of MIR181A2HG expression compared to normal tissue. The overexpression of MIR181A2HG resulted in the inhibition of HaCaT keratinocytes proliferation. The knockdown of MIR181A2HG promoted cell proliferation. The dual-luciferase reporter assay and rescue experiments provided evidence of the interaction among MIR181A2HG, SOX6, and miR-223-3p.
Conclusions: The lncRNA MIR181A2HG functions as a miR-223-3p sponge targeting SOX6 to regulate the proliferation of keratinocytes, which suggested that MIR181A2HG/miR-223-3p/SOX6 might be potential diagnostic and therapeutic targets for psoriasis.
Keywords: MIR181A2HG; SOX6; keratinocytes; miR-223-3p; psoriasis.
Conflict of interest statement
Figures





Similar articles
-
LncRNA MIR181A2HG negatively regulates human keratinocytes proliferation by binding SRSF1.Cytotechnology. 2024 Jun;76(3):313-327. doi: 10.1007/s10616-024-00621-6. Epub 2024 Mar 26. Cytotechnology. 2024. PMID: 38736729 Free PMC article.
-
The lncRNA H19/miR-766-3p/S1PR3 Axis Contributes to the Hyperproliferation of Keratinocytes and Skin Inflammation in Psoriasis via the AKT/mTOR Pathway.Mediators Inflamm. 2021 Dec 28;2021:9991175. doi: 10.1155/2021/9991175. eCollection 2021. Mediators Inflamm. 2021. PMID: 34992498 Free PMC article.
-
MiR-193b-3p-ERBB4 axis regulates psoriasis pathogenesis via modulating cellular proliferation and inflammatory-mediator production of keratinocytes.Cell Death Dis. 2021 Oct 19;12(11):963. doi: 10.1038/s41419-021-04230-5. Cell Death Dis. 2021. PMID: 34667159 Free PMC article.
-
Sox6, A Potential Target for MicroRNAs in Cardiometabolic Disease.Curr Hypertens Rep. 2022 May;24(5):145-156. doi: 10.1007/s11906-022-01175-8. Epub 2022 Feb 5. Curr Hypertens Rep. 2022. PMID: 35124768 Review.
-
Sox6, jack of all trades: a versatile regulatory protein in vertebrate development.Dev Dyn. 2011 Jun;240(6):1311-21. doi: 10.1002/dvdy.22639. Epub 2011 Apr 14. Dev Dyn. 2011. PMID: 21495113 Free PMC article. Review.
Cited by
-
m6A- and m5C- modified lncRNAs orchestrate the prognosis in cutaneous melanoma and m6A- modified LINC00893 regulates cutaneous melanoma cell metastasis.Skin Res Technol. 2024 Jul;30(7):e13842. doi: 10.1111/srt.13842. Skin Res Technol. 2024. PMID: 38965799 Free PMC article.
-
Long Non-Coding RNAs in Psoriasis and Cutaneous Squamous Cell Carcinoma.J Clin Med. 2025 Jul 17;14(14):5081. doi: 10.3390/jcm14145081. J Clin Med. 2025. PMID: 40725772 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

