Mechanism of human PINK1 activation at the TOM complex in a reconstituted system
- PMID: 38848361
- PMCID: PMC11160474
- DOI: 10.1126/sciadv.adn7191
Mechanism of human PINK1 activation at the TOM complex in a reconstituted system
Abstract
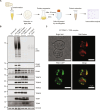
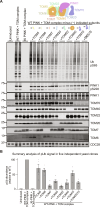
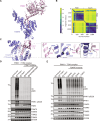
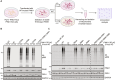
Loss-of-function mutations in PTEN-induced kinase 1 (PINK1) are a frequent cause of early-onset Parkinson's disease (PD). Stabilization of PINK1 at the translocase of outer membrane (TOM) complex of damaged mitochondria is critical for its activation. The mechanism of how PINK1 is activated in the TOM complex is unclear. Here, we report that co-expression of human PINK1 and all seven TOM subunits in Saccharomyces cerevisiae is sufficient for PINK1 activation. We use this reconstitution system to systematically assess the role of each TOM subunit toward PINK1 activation. We unambiguously demonstrate that the TOM20 and TOM70 receptor subunits are required for optimal PINK1 activation and map their sites of interaction with PINK1 using AlphaFold structural modeling and mutagenesis. We also demonstrate an essential role of the pore-containing subunit TOM40 and its structurally associated subunits TOM7 and TOM22 for PINK1 activation. These findings will aid in the development of small-molecule activators of PINK1 as a therapeutic strategy for PD.
Figures


References
-
- Valente E. M., Abou-Sleiman P. M., Caputo V., Muqit M. M. K., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A. R., Healy D. G., Albanese A., Nussbaum R., González-Maldonado R., Deller T., Salvi S., Cortelli P., Gilks W. P., Latchman D. S., Harvey R. J., Dallapiccola B., Auburger G., Wood N. W., Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304, 1158–1160 (2004). - PubMed
-
- Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N., Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998). - PubMed
-
- Agarwal S., Muqit M. M. K., PTEN-induced kinase 1 (PINK1) and Parkin: Unlocking a mitochondrial quality control pathway linked to Parkinson’s disease. Curr. Opin. Neurobiol. 72, 111–119 (2022). - PubMed
-
- Vargas J. N. S., Hamasaki M., Kawabata T., Youle R. J., Yoshimori T., The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 24, 167–185 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

