Nuclear factor κB overactivation in the intervertebral disc leads to macrophage recruitment and severe disc degeneration
- PMID: 38848366
- PMCID: PMC11160472
- DOI: 10.1126/sciadv.adj3194
Nuclear factor κB overactivation in the intervertebral disc leads to macrophage recruitment and severe disc degeneration
Abstract
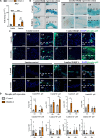
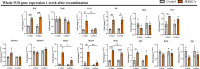
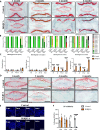
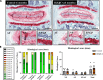
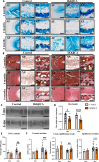
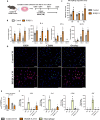
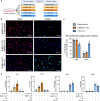
Persistent inflammation has been associated with severe disc degeneration (DD). This study investigated the effect of prolonged nuclear factor κB (NF-κB) activation in DD. Using an inducible mouse model, we genetically targeted cells expressing aggrecan, a primary component of the disc extra cellular matrix, for activation of the canonical NF-κB pathway. Prolonged NF-κB activation led to severe structural degeneration accompanied by increases in gene expression of inflammatory molecules (Il1b, Cox2, Il6, and Nos2), chemokines (Mcp1 and Mif), and catabolic enzymes (Mmp3, Mmp9, and Adamts4). Increased recruitment of proinflammatory (F4/80+,CD38+) and inflammatory resolving (F4/80+,CD206+) macrophages was observed within caudal discs. We found that the secretome of inflamed caudal disc cells increased macrophage migration and inflammatory activation. Lumbar discs did not exhibit phenotypic changes, suggestive of regional spinal differences in response to inflammatory genetic overactivation. Results suggest prolonged NF-κB activation can induce severe DD through increases in inflammatory cytokines, chemotactic proteins, catabolic enzymes, and the recruitment and activation of macrophage cell populations.
Figures


Update of
-
Nuclear Factor Kappa B Over-Activation in the Intervertebral Disc Leads to Macrophage Recruitment and Severe Disc Degeneration.bioRxiv [Preprint]. 2023 Aug 8:2023.08.07.552274. doi: 10.1101/2023.08.07.552274. bioRxiv. 2023. Update in: Sci Adv. 2024 Jun 7;10(23):eadj3194. doi: 10.1126/sciadv.adj3194. PMID: 37609194 Free PMC article. Updated. Preprint.
References
-
- Murray C. J., Atkinson C., Bhalla K., Birbeck G., Burstein R., Chou D., Dellavalle R., Danaei G., Ezzati M., Fahimi A., Flaxman D., Foreman, Gabriel S., Gakidou E., Kassebaum N., Khatibzadeh S., Lim S., Lipshultz S. E., London S., Lopez, MacIntyre M., Mokdad A. H., Moran A., Moran A. E., Mozaffarian D., Murphy T., Naghavi M., Pope C., Roberts T., Salomon J., Schwebel D. C., Shahraz S., Sleet D. A., Murray, Abraham J., Ali M. K., Atkinson C., Bartels D. H., Bhalla K., Birbeck G., Burstein R., Chen H., Criqui M. H., Dahodwala, Jarlais, Ding E. L., Dorsey E. R., Ebel B. E., Ezzati M., Fahami, Flaxman S., Flaxman A. D., Gonzalez-Medina D., Grant B., Hagan H., Hoffman H., Kassebaum N., Khatibzadeh S., Leasher J. L., Lin J., Lipshultz S. E., Lozano R., Lu Y., Mallinger L., McDermott M., Micha R., Miller T. R., Mokdad A. A., Mokdad A. H., Mozaffarian D., Naghavi M., Narayan K. M., Omer S. B., Pelizzari P. M., Phillips D., Ranganathan D., Rivara F. P., Roberts T., Sampson U., Sanman E., Sapkota A., Schwebel D. C., Sharaz S., Shivakoti R., Singh G. M., Singh D., Tavakkoli M., Towbin J. A., Wilkinson J. D., Zabetian A., Murray, Abraham J., Ali M. K., Alvardo M., Atkinson C., Baddour L. M., Benjamin E. J., Bhalla K., Birbeck G., Bolliger I., Burstein R., Carnahan E., Chou D., Chugh S. S., Cohen A., Colson K. E., Cooper L. T., Couser W., Criqui M. H., Dabhadkar K. C., Dellavalle R. P., Jarlais, Dicker D., Dorsey E. R., Duber H., Ebel B. E., Engell R. E., Ezzati M., Felson D. T., Finucane M. M., Flaxman S., Flaxman A. D., Fleming T., Foreman, Forouzanfar M. H., Freedman G., Freeman M. K., Gakidou E., Gillum R. F., Gonzalez-Medina D., Gosselin R., Gutierrez H. R., Hagan H., Havmoeller R., Hoffman H., Jacobsen K. H., James S. L., Jasrasaria R., Jayarman S., Johns N., Kassebaum N., Khatibzadeh S., Lan Q., Leasher J. L., Lim S., Lipshultz S. E., London S., Lopez, Lozano R., Lu Y., Mallinger L., Meltzer M., Mensah G. A., Michaud C., Miller T. R., Mock C., Moffitt T. E., Mokdad A. A., Mokdad A. H., Moran A., Naghavi M., Narayan K. M., Nelson R. G., Olives C., Omer S. B., Ortblad K., Ostro B., Pelizzari P. M., Phillips D., Raju M., Razavi H., Ritz B., Roberts T., Sacco R. L., Salomon J., Sampson U., Schwebel D. C., Shahraz S., Shibuya K., Silberberg D., Singh J. A., Steenland K., Taylor J. A., Thurston G. D., Vavilala M. S., Vos T., Wagner G. R., Weinstock M. A., Weisskopf M. G., Wulf S., Murray, U.S. Burden of Disease Collaborators , The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA 310, 591–608 (2013). - PMC - PubMed
-
- Katz J. N., Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J. Bone Joint Surg. Am. 88, 21–24 (2006). - PubMed
-
- Luoma K., Riihimäki H., Luukkonen R., Raininko R., Viikari-Juntura E., Lamminen A., Low back pain in relation to lumbar disc degeneration. Spine 25, 487–492 (2000). - PubMed
-
- Nerlich A. G., Bachmeier B. E., Schleicher E., Rohrbach H., Paesold G., Boos N., Immunomorphological analysis of rage receptor expression and nf-κb activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann. N. Y. Acad. Sci. 1096, 239–248 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

