Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial
- PMID: 38848477
- PMCID: PMC11161857
- DOI: 10.1001/jamainternmed.2024.2007
Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial
Erratum in
-
Errors in Figure 2.JAMA Intern Med. 2024 Sep 1;184(9):1137. doi: 10.1001/jamainternmed.2024.3735. JAMA Intern Med. 2024. PMID: 39037781 Free PMC article. No abstract available.
Abstract
Importance: There is an urgent need to identify treatments for postacute sequelae of SARS-CoV-2 infection (PASC).
Objective: To assess the efficacy of a 15-day course of nirmatrelvir-ritonavir in reducing the severity of select PASC symptoms.
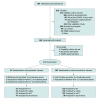
Design, setting, and participants: This was a 15-week blinded, placebo-controlled, randomized clinical trial conducted from November 2022 to September 2023 at Stanford University (California). The participants were adults with moderate to severe PASC symptoms of 3 months or longer duration.
Interventions: Participants were randomized 2:1 to treatment with oral nirmatrelvir-ritonavir (NMV/r, 300 mg and 100 mg) or with placebo-ritonavir (PBO/r) twice daily for 15 days.
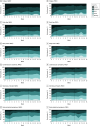
Main outcomes and measures: Primary outcome was a pooled severity of 6 PASC symptoms (fatigue, brain fog, shortness of breath, body aches, gastrointestinal symptoms, and cardiovascular symptoms) based on a Likert scale score at 10 weeks. Secondary outcomes included symptom severity at different time points, symptom burden and relief, patient global measures, Patient-Reported Outcomes Measurement Information System (PROMIS) measures, orthostatic vital signs, and sit-to-stand test change from baseline.
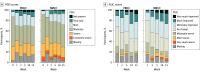
Results: Of the 155 participants (median [IQR] age, 43 [34-54] years; 92 [59%] females), 102 were randomized to the NMV/r group and 53 to the PBO/r group. Nearly all participants (n = 153) had received the primary series for COVID-19 vaccination. Mean (SD) time between index SARS-CoV-2 infection and randomization was 17.5 (9.1) months. There was no statistically significant difference in the model-derived severity outcome pooled across the 6 core symptoms at 10 weeks between the NMV/r and PBO/r groups. No statistically significant between-group differences were found at 10 weeks in the Patient Global Impression of Severity or Patient Global Impression of Change scores, summative symptom scores, and change from baseline to 10 weeks in PROMIS fatigue, dyspnea, cognitive function, and physical function measures. Adverse event rates were similar in NMV/r and PBO/r groups and mostly of low grade.
Conclusions and relevance: The results of this randomized clinical trial showed that a 15-day course of NMV/r in a population of patients with PASC was generally safe but did not demonstrate a significant benefit for improving select PASC symptoms in a mostly vaccinated cohort with protracted symptom duration. Further studies are needed to determine the role of antivirals in the treatment of PASC.
Trial registration: ClinicalTrials.gov Identifier: NCT05576662.
Conflict of interest statement
Figures
References
-
- Wulf Hanson S, Abbafati C, Aerts JG, et al. ; Global Burden of Disease Long COVID Collaborators . Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604-1615. doi: 10.1001/jama.2022.18931 - DOI - PMC - PubMed
-
- US Centers for Disease Control and Prevention . Long COVID-19—Household Pulse Survey—COVID-19. Published October 11, 2023. Accessed October 18, 2023. https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

