Biomimetic Ghost Nanomedicine-Based Optotheranostics for Cancer
- PMID: 38848540
- PMCID: PMC11247544
- DOI: 10.1021/acs.nanolett.4c01534
Biomimetic Ghost Nanomedicine-Based Optotheranostics for Cancer
Abstract
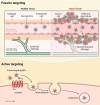
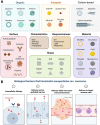
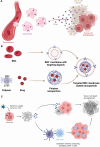
Theranostic medicine combines diagnostics and therapeutics, focusing on solid tumors at minimal doses. Optically activated photosensitizers are significant examples owing to their photophysical and chemical properties. Several optotheranostics have been tested that convert light to imaging signals, therapeutic radicals, and heat. Upon light exposure, conjugated photosensitizers kill tumor cells by producing reactive oxygen species and heat or by releasing cancer antigens. Despite clinical trials, these molecularly conjugated photosensitizers require protection from their surroundings and a localized direction for site-specific delivery during blood circulation. Therefore, cell membrane biomimetic ghosts have been proposed for precise and safe delivery of these optically active large molecules, which are clinically relevant because of their biocompatibility, long circulation time, bypass of immune cell recognition, and targeting ability. This review focuses on the role of biomimetic nanoparticles in the treatment and diagnosis of tumors through light-mediated diagnostics and therapy, providing insights into their preclinical and clinical status.
Keywords: Biomimetics; Cell Ghosts; Optotheranostics; Phototherapeutics; Solid tumors.
Conflict of interest statement
The authors declare the following competing financial interest(s): J.C. is a co-founder and shareholder of TargTex S.A -Targeted Therapeutics for Glioblastoma Multiforme. J.C. is a member of the Global Burden Disease (GBD) consortium of the Institute for Health Metrics and Evaluation (IHME), University of Washington (US), and the Scientific Advisory Board of Vector Bioscience, Cambridge. R.P. holds patents for liposomes and lipid-based nanoparticles. The other authors declare no competing financial interests and have approved the final submission.
Figures


Similar articles
-
Innovative approaches for cancer treatment: graphene quantum dots for photodynamic and photothermal therapies.J Mater Chem B. 2024 May 8;12(18):4307-4334. doi: 10.1039/d4tb00255e. J Mater Chem B. 2024. PMID: 38595268 Review.
-
A biomimetic camouflaged metal organic framework for enhanced siRNA delivery in the tumor environment.J Mater Chem B. 2024 May 1;12(17):4080-4096. doi: 10.1039/d3tb02827e. J Mater Chem B. 2024. PMID: 38577851 Review.
-
Tissue-Resident Macrophage Membrane-Coated Nanomedicine for Targeted Tumor Therapy.ACS Nano. 2025 Jul 29;19(29):26296-26319. doi: 10.1021/acsnano.5c04463. Epub 2025 Jul 18. ACS Nano. 2025. PMID: 40680020 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
One-pot synthesis of conjugated small molecules for construction of NIR fluorescence/photoacoustic dual-modal imaging nanoagents with improved photothermal/photodynamic/chemodynamic effects.J Photochem Photobiol B. 2025 Aug;269:113204. doi: 10.1016/j.jphotobiol.2025.113204. Epub 2025 Jun 23. J Photochem Photobiol B. 2025. PMID: 40592079
Cited by
-
Research progress of novel anti-tumor drug formulations.Front Oncol. 2024 Dec 16;14:1507958. doi: 10.3389/fonc.2024.1507958. eCollection 2024. Front Oncol. 2024. PMID: 39737395 Free PMC article. Review.
-
Preferential activation of type I interferon-mediated antitumor inflammatory signaling by CuS/MnO2/diAMP nanoparticles enhances anti-PD-1 therapy for sporadic colorectal cancer.J Nanobiotechnology. 2024 Nov 12;22(1):699. doi: 10.1186/s12951-024-02970-y. J Nanobiotechnology. 2024. PMID: 39533269 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

