The expanding clinical and genetic spectrum of DYNC1H1-related disorders
- PMID: 38848546
- PMCID: PMC11788221
- DOI: 10.1093/brain/awae183
The expanding clinical and genetic spectrum of DYNC1H1-related disorders
Abstract
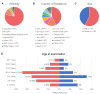
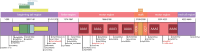
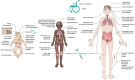
Intracellular trafficking involves an intricate machinery of motor complexes, including the dynein complex, to shuttle cargo for autophagolysosomal degradation. Deficiency in dynein axonemal chains, as well as cytoplasmic light and intermediate chains, have been linked with ciliary dyskinesia and skeletal dysplasia. The cytoplasmic dynein 1 heavy chain protein (DYNC1H1) serves as a core complex for retrograde trafficking in neuronal axons. Dominant pathogenic variants in DYNC1H1 have been previously implicated in peripheral neuromuscular disorders (NMD) and neurodevelopmental disorders (NDD). As heavy-chain dynein is ubiquitously expressed, the apparent selectivity of heavy chain dyneinopathy for motor neuronal phenotypes remains currently unaccounted for. Here, we aimed to evaluate the full DYNC1H1-related clinical, molecular and imaging spectrum, including multisystem features and novel phenotypes presenting throughout life. We identified 47 cases from 43 families with pathogenic heterozygous variants in DYNC1H1 (aged 0-59 years) and collected phenotypic data via a comprehensive standardized survey and clinical follow-up appointments. Most patients presented with divergent and previously unrecognized neurological and multisystem features, leading to significant delays in genetic testing and establishing the correct diagnosis. Neurological phenotypes include novel autonomic features, previously rarely described behavioral disorders, movement disorders and periventricular lesions. Sensory neuropathy was identified in nine patients (median age of onset 10.6 years), of which five were only diagnosed after the second decade of life, and three had a progressive age-dependent sensory neuropathy. Novel multisystem features included primary immunodeficiency, bilateral sensorineural hearing loss, organ anomalies and skeletal manifestations, resembling the phenotypic spectrum of other dyneinopathies. We also identified an age-dependent biphasic disease course with developmental regression in the first decade and, following a period of stability, neurodegenerative progression after the second decade of life. Of note, we observed several cases in whom neurodegeneration appeared to be prompted by intercurrent systemic infections with double-stranded DNA viruses (Herpesviridae) or single-stranded RNA viruses (Ross River fever, SARS-CoV-2). Moreover, the disease course appeared to be exacerbated by viral infections regardless of age and/or severity of neurodevelopmental disorder manifestations, indicating a role of dynein in anti-viral immunity and neuronal health. In summary, our findings expand the clinical, imaging and molecular spectrum of pathogenic DYNC1H1 variants beyond motor neuropathy disorders and suggest a life-long continuum and age-related progression due to deficient intracellular trafficking. This study will facilitate early diagnosis and improve counselling and health surveillance of affected patients.
Keywords: autophagy; intracellular trafficking; neurodevelopmental disorders; viral immunity.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. - PubMed
MeSH terms
Substances
Grants and funding
- Broad Institute of MIT and Harvard
- UM1 HG008900/HG/NHGRI NIH HHS/United States
- German Society for Muscle Diseases
- P50 HD105351/HD/NICHD NIH HHS/United States
- EY/NEI NIH HHS/United States
- UM1HG008900/HL/NHLBI NIH HHS/United States
- AZV NU20-04-00279/Ministry of Health of the Czech Republic
- NS/NINDS NIH HHS/United States
- European Union Horizon 2020 Programme
- 2446/Action Medical Research
- Cologne Clinician Scientist Program/Medical Faculty/University of Cologne and German Research Foundation
- P50HD105351/Boston Children's Hospital IDDRC Molecular Genetics Core Facility
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

