Intranasal SARS-CoV-2 Omicron variant vaccines elicit humoral and cellular mucosal immunity in female mice
- PMID: 38848648
- PMCID: PMC11200293
- DOI: 10.1016/j.ebiom.2024.105185
Intranasal SARS-CoV-2 Omicron variant vaccines elicit humoral and cellular mucosal immunity in female mice
Abstract
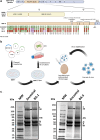
Background: In order to prevent the emergence and spread of future variants of concern of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), developing vaccines capable of stopping transmission is crucial. The SARS-CoV-2 vaccine NDV-HXP-S can be administered live intranasally (IN) and thus induce protective immunity in the upper respiratory tract. The vaccine is based on Newcastle disease virus (NDV) expressing a stabilised SARS-CoV-2 spike protein. NDV-HXP-S can be produced as influenza virus vaccine at low cost in embryonated chicken eggs.
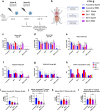
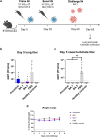
Methods: The NDV-HXP-S vaccine was genetically engineered to match the Omicron variants of concern (VOC) BA.1 and BA.5 and tested as an IN two or three dose vaccination regimen in female mice. Furthermore, female mice intramuscularly (IM) vaccinated with mRNA-lipid nanoparticles (LNPs) were IN boosted with NDV-HXP-S. Systemic humoral immunity, memory T cell responses in the lungs and spleens as well as immunoglobulin A (IgA) responses in distinct mucosal tissues were characterised.
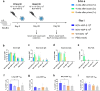
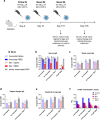
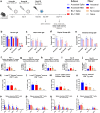
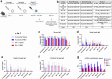
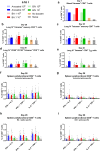
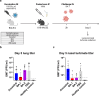
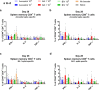
Findings: NDV-HXP-S Omicron variant vaccines elicited high mucosal IgA and serum IgG titers against respective SARS-CoV-2 VOC in female mice following IN administration and protected against challenge from matched variants. Additionally, antigen-specific memory B cells and local T cell responses in the lungs were induced. Host immunity against the NDV vector did not interfere with boosting. Intramuscular vaccination with mRNA-LNPs was enhanced by IN NDV-HXP-S boosting resulting in improvement of serum neutralization titers and induction of mucosal immunity.
Interpretation: We demonstrate that NDV-HXP-S Omicron variant vaccines utilised for primary immunizations or boosting efficiently elicit humoral and cellular immunity. The described induction of systemic and mucosal immunity has the potential to reduce infection and transmission.
Funding: This work was partially funded by the NIAIDCenters of Excellence for Influenza Research and Response (CEIRR) and by the NIAID Collaborative Vaccine Innovation Centers and by institutional funding from the Icahn School of Medicine at Mount Sinai. See under Acknowledgements for details.
Keywords: COVID-19; Low cost vaccine platform; Mucosal immune response; NDV vector; Prime-pull vaccination; Variant vaccine; mRNA vaccine.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The Icahn School of Medicine at Mount Sinai has filed patent applications entitled “RECOMBINANT NEWCASTLE DISEASE VIRUS EXPRESSING SARS-COV-2 SPIKE PROTEIN AND USES THEREOF” which names P.P., A.G.S, F.K. and W.S. as inventors. Mount Sinai is seeking to commercialise this vaccine; therefore, the institution and its faculty inventors could benefit financially. I.G.D. has co-chaired at the ninth ESWI Influenza conference, which has no competing interest with this work. The M.S. laboratory has received unrelated research funding in sponsored research agreements from 7Hills Pharma, ArgenX N.V., Moderna and Phio Pharmaceuticals, which has no competing interest with this work. F.K. has consulted for Merck, Seqirus, Curevac and Pfizer, and is currently consulting for Pfizer, Third Rock Ventures, GSK and Avimex. The FK laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. The A.G.-S. laboratory has received research support from GSK, Pfizer, Senhwa Biosciences, Kenall Manufacturing, Blade Therapeutics, Avimex, Johnson & Johnson, Dynavax, 7Hills Pharma, Pharmamar, ImmunityBio, Accurius, Nanocomposix, Hexamer, N-fold LLC, Model Medicines, Atea Pharma, Applied Biological Laboratories and Merck. A.G.S. has consulting agreements for the following companies involving cash and/or stock: Castlevax, Amovir, Vivaldi Biosciences, Contrafect, 7Hills Pharma, Avimex, Pagoda, Accurius, Esperovax, Farmak, Applied Biological Laboratories, Pharmamar, CureLab Oncology, CureLab Veterinary, Synairgen, Paratus and Pfizer. A.G.S. has been an invited speaker in meeting events organised by Seqirus, Janssen, Abbott and Astrazeneca. A.G.S. is inventor on patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections and cancer, owned by the Icahn School of Medicine at Mount Sinai, New York. Specifically, A.G.S., a member of the faculty of the Icahn School of Medicine at Mount Sinai (Mount Sinai) is an inventor of a novel COVID-19 vaccine currently being investigated in clinical trials. Mount Sinai is advancing the development of this vaccine and related technologies for potential commercial use. Mount Sinai has created CastleVax Inc., a Mount Sinai company, and has licensed the applicable IP to it. Mount Sinai will receive financial compensation from CastleVax Inc. pursuant to that license if vaccine development proceeds and as an owner of the company subject to the sale of its ownership interest in the future. Subject to Mount Sinai receiving such financial consideration, A.G.S. will receive a portion of that consideration pursuant to the terms of the Mount Sinai Intellectual Property Policy. All other authors declare no competing interests.
Figures



Similar articles
-
Mucosal multivalent NDV-based vaccine provides cross-reactive immune responses against SARS-CoV-2 variants in animal models.Front Immunol. 2025 Mar 17;16:1524477. doi: 10.3389/fimmu.2025.1524477. eCollection 2025. Front Immunol. 2025. PMID: 40165947 Free PMC article.
-
A single immunization with intranasal Newcastle disease virus (NDV)-based XBB.1.5 variant vaccine reduces disease and transmission in animals against matched-variant challenge.Vaccine. 2025 Jan 25;45:126586. doi: 10.1016/j.vaccine.2024.126586. Epub 2024 Dec 12. Vaccine. 2025. PMID: 39667115
-
Safety and Immunogenicity Analysis of a Newcastle Disease Virus (NDV-HXP-S) Expressing the Spike Protein of SARS-CoV-2 in Sprague Dawley Rats.Front Immunol. 2021 Nov 18;12:791764. doi: 10.3389/fimmu.2021.791764. eCollection 2021. Front Immunol. 2021. PMID: 34868082 Free PMC article.
-
T and B cell responses in different immunization scenarios for COVID-19: a narrative review.Front Immunol. 2025 Mar 18;16:1535014. doi: 10.3389/fimmu.2025.1535014. eCollection 2025. Front Immunol. 2025. PMID: 40170841 Free PMC article. Review.
-
Upper respiratory tract mucosal immunity for SARS-CoV-2 vaccines.Trends Mol Med. 2023 Apr;29(4):255-267. doi: 10.1016/j.molmed.2023.01.003. Epub 2023 Jan 23. Trends Mol Med. 2023. PMID: 36764906 Free PMC article. Review.
Cited by
-
Evaluation of the immune responses of biological adjuvant bivalent vaccine with three different insertion modes for ND and IBD.Virulence. 2024 Dec;15(1):2387181. doi: 10.1080/21505594.2024.2387181. Epub 2024 Aug 5. Virulence. 2024. PMID: 39101682 Free PMC article.
-
Comparative Efficacy of Parenteral and Mucosal Recombinant Probiotic Vaccines Against SARS-CoV-2 and S. pneumoniae Infections in Animal Models.Vaccines (Basel). 2024 Oct 19;12(10):1195. doi: 10.3390/vaccines12101195. Vaccines (Basel). 2024. PMID: 39460360 Free PMC article.
-
Mucosal multivalent NDV-based vaccine provides cross-reactive immune responses against SARS-CoV-2 variants in animal models.Front Immunol. 2025 Mar 17;16:1524477. doi: 10.3389/fimmu.2025.1524477. eCollection 2025. Front Immunol. 2025. PMID: 40165947 Free PMC article.
-
A Surrogate Enzyme-Linked Immunosorbent Assay to Select High-Titer Human Convalescent Plasma for Treating Immunocompromised Patients Infected With Severe Acute Respiratory Syndrome Coronavirus 2 Variants of Concern.J Infect Dis. 2025 Apr 15;231(4):e723-e733. doi: 10.1093/infdis/jiae645. J Infect Dis. 2025. PMID: 39749487 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

