The Heat Shock Response as a Condensate Cascade
- PMID: 38848866
- PMCID: PMC11214683
- DOI: 10.1016/j.jmb.2024.168642
The Heat Shock Response as a Condensate Cascade
Abstract
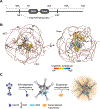
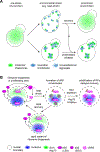
The heat shock response (HSR) is a gene regulatory program controlling expression of molecular chaperones implicated in aging, cancer, and neurodegenerative disease. Long presumed to be activated by toxic protein aggregates, recent work suggests a new functional paradigm for the HSR in yeast. Rather than toxic aggregates, adaptive biomolecular condensates comprised of orphan ribosomal proteins (oRP) and stress granule components have been shown to be physiological chaperone clients. By titrating away the chaperones Sis1 and Hsp70 from the transcription factor Hsf1, these condensates activate the HSR. Upon release from Hsp70, Hsf1 forms spatially distinct transcriptional condensates that drive high expression of HSR genes. In this manner, the negative feedback loop controlling HSR activity - in which Hsf1 induces Hsp70 expression and Hsp70 represses Hsf1 activity - is embedded in the biophysics of the system. By analogy to phosphorylation cascades that transmit information via the dynamic activity of kinases, we propose that the HSR is organized as a condensate cascade that transmits information via the localized activity of molecular chaperones.
Keywords: Condensate; Heat shock; Hsf1; Hsp70; Signaling cascade.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

