BIGH3 mediates apoptosis and gap junction failure in osteocytes during renal cell carcinoma bone metastasis progression
- PMID: 38849015
- PMCID: PMC11964150
- DOI: 10.1016/j.canlet.2024.217009
BIGH3 mediates apoptosis and gap junction failure in osteocytes during renal cell carcinoma bone metastasis progression
Abstract
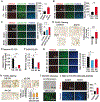
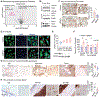
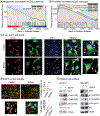
Renal cell carcinoma (RCC) bone metastatis progression is driven by crosstalk between tumor cells and the bone microenvironment, which includes osteoblasts, osteoclasts, and osteocytes. RCC bone metastases (RCCBM) are predominantly osteolytic and resistant to antiresorptive therapy. The molecular mechanisms underlying pathologic osteolysis and disruption of bone homeostasis remain incompletely understood. We previously reported that BIGH3/TGFBI (transforming growth factor-beta-induced protein ig-h3, shortened to BIGH3 henceforth) secreted by colonizing RCC cells drives osteolysis by inhibiting osteoblast differentiation, impairing healing of osteolytic lesions, which is reversible with osteoanabolic agents. Here, we report that BIGH3 induces osteocyte apoptosis in both human RCCBM tissue specimens and in a preclinical mouse model. We also demonstrate that BIGH3 reduces Cx43 expression, blocking gap junction (GJ) function and osteocyte network communication. BIGH3-mediated GJ inhibition is blocked by the lysosomal inhibitor hydroxychloroquine (HCQ), but not osteoanabolic agents. Our results broaden the understanding of pathologic osteolysis in RCCBM and indicate that targeting the BIGH3 mechanism could be a combinational strategy for the treatment of RCCBM-induced bone disease that overcomes the limited efficacy of antiresorptives that target osteoclasts.
Keywords: Apoptosis; BIGH3/TGFBI; Bone metastasis; Osteocyte; Renal cell carcinoma.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



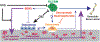
References
-
- Wood SL, Brown JE, Skeletal metastasis in renal cell carcinoma: current and future management options, Cancer Treat Rev. 38 (2012) 284–291. - PubMed
-
- Pan T, Lin SC, Yu KJ, Yu G, Song JH, Lewis VO, Bird JE, Moon B, Lin PP, Tannir NM, Jonasch E, Wood CG, Gallick GE, Yu-Lee LY, Lin SH, Satcher RL, BIGH3 promotes osteolytic lesions in renal cell carcinoma bone metastasis by inhibiting osteoblast differentiation, Neoplasia 20 (2018) 32–43. - PMC - PubMed
-
- Skonier J, Bennett K, Rothwell V, Kosowski S, Plowman G, Wallace P, Edelhoff S, Disteche C, Neubauer M, Marquardt H, et al., Beta ig-h3: a transforming growth factor-beta-responsive gene encoding a secreted protein that inhibits cell attachment in vitro and suppresses the growth of CHO cells in nude mice, DNA Cell Biol. 13 (1994) 571–584. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

