Carbon dots as dual inhibitors of tau and amyloid-beta aggregation for the treatment of Alzheimer's disease
- PMID: 38849023
- PMCID: PMC11368047
- DOI: 10.1016/j.actbio.2024.06.001
Carbon dots as dual inhibitors of tau and amyloid-beta aggregation for the treatment of Alzheimer's disease
Abstract
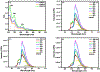
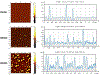
Alzheimer's disease (AD) is the most common form of senile dementia, presenting a significant challenge for the development of effective treatments. AD is characterized by extracellular amyloid plaques and intraneuronal neurofibrillary tangles. Therefore, targeting both hallmarks through inhibition of amyloid beta (Aβ) and tau aggregation presents a promising approach for drug development. Carbon dots (CD), with their high biocompatibility, minimal cytotoxicity, and blood-brain barrier (BBB) permeability, have emerged as promising drug nanocarriers. Congo red, an azo dye, has gathered significant attention for inhibiting amyloid-beta and tau aggregation. However, Congo red's inability to cross the BBB limits its potential to be used as a drug candidate for central nervous system (CNS) diseases. Furthermore, current studies only focus on using Congo red to target single disease hallmarks, without investigating dual inhibition capabilities. In this study, we synthesized Congo red-derived CD (CRCD) by using Congo red and citric acid as precursors, resulting in three variants, CRCD1, CRCD2 and CRCD3, based on different mass ratios of precursors. CRCD2 and CRCD3 exhibited sustained low cytotoxicity, and CRCD3 demonstrated the ability to traverse the BBB in a zebrafish model. Moreover, thioflavin T (ThT) aggregation assays and AFM imaging revealed CRCD as potent inhibitors against both tau and Aβ aggregation. Notably, CRCD1 emerged as the most robust inhibitor, displaying IC50 values of 0.2 ± 0.1 and 2.1 ± 0.5 µg/mL against tau and Aβ aggregation, respectively. Our findings underscore the dual inhibitory role of CRCD against tau and Aβ aggregation, showcasing effective BBB penetration and positioning CRCD as potential nanodrugs and nanocarriers for the CNS. Hence, CRCD-based compounds represent a promising candidate in the realm of multi-functional AD therapeutics, offering an innovative formulation component for future developments in this area. STATEMENT OF SIGNIFICANCE: This article reports Congo red-derived carbon dots (CRCD) as dual inhibitors of tau and amyloid-beta (Aβ) aggregation for the treatment of Alzheimer's disease (AD). The CRCD are biocompatible and show strong fluorescence, high stability, the ability to cross the blood-brain barrier, and the function of addressing two major pathological features of AD.
Keywords: Alzheimer's disease; Blood-brain barrier; Carbon dots; Congo red; Dual inhibitors.
Copyright © 2024 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing financial interest.
Figures





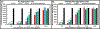





Similar articles
-
Design, synthesis, and biological evaluation of tetrahydropyrimidine analogue as GSK-3β/Aβ aggregation inhibitor and anti-Alzheimer's agent.Bioorg Chem. 2024 Dec;153:107811. doi: 10.1016/j.bioorg.2024.107811. Epub 2024 Sep 7. Bioorg Chem. 2024. PMID: 39270527
-
Hyperphosphorylated tau-based Alzheimer's Disease drug discovery: Identification of inhibitors of tau aggregation and cytotoxicity.SLAS Discov. 2025 Jun;33:100235. doi: 10.1016/j.slasd.2025.100235. Epub 2025 May 3. SLAS Discov. 2025. PMID: 40319815 Free PMC article.
-
In Silico Investigation of Ganoderic Acid A Targeting Amyloid-Beta and Tau Protein Aggregation in Alzheimer's Disease.Int J Med Mushrooms. 2025;27(9):85-92. doi: 10.1615/IntJMedMushrooms.2025059137. Int J Med Mushrooms. 2025. PMID: 40743720
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
Dysfunction of the blood-brain barrier in Alzheimer's disease: Evidence from human studies.Neuropathol Appl Neurobiol. 2022 Apr;48(3):e12782. doi: 10.1111/nan.12782. Epub 2022 Feb 2. Neuropathol Appl Neurobiol. 2022. PMID: 34823269
Cited by
-
Nanoscale drug formulations for the treatment of Alzheimer's disease progression.RSC Adv. 2025 Feb 7;15(6):4031-4078. doi: 10.1039/d4ra08128e. eCollection 2025 Feb 6. RSC Adv. 2025. PMID: 39926227 Free PMC article. Review.
-
Dual modulation of amyloid beta and tau aggregation and dissociation in Alzheimer's disease: a comprehensive review of the characteristics and therapeutic strategies.Transl Neurodegener. 2025 Mar 26;14(1):15. doi: 10.1186/s40035-025-00479-4. Transl Neurodegener. 2025. PMID: 40133924 Free PMC article. Review.
-
Advancing Nanomaterial-Based Strategies for Alzheimer's Disease: A Perspective.JACS Au. 2025 Mar 31;5(4):1519-1537. doi: 10.1021/jacsau.5c00002. eCollection 2025 Apr 28. JACS Au. 2025. PMID: 40313833 Free PMC article. Review.
-
Fluorescent carbon dots in PEC-GS/BG hybrids and their application for bioimaging.Front Mol Biosci. 2025 Jun 19;12:1555995. doi: 10.3389/fmolb.2025.1555995. eCollection 2025. Front Mol Biosci. 2025. PMID: 40612059 Free PMC article.
References
-
- Dubois B, Villain N, Frisoni GB, Rabinovici GD, Sabbagh M, Cappa S, Bejanin A, Bombois S, Epelbaum S, Teichmann M, Habert M-O, Nordberg A, Blennow K, Galasko D, Stern Y, Rowe CC, Salloway S, Schneider LS, Cummings JL, Feldman HH, Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group, Lancet Neurol. 20 (2021) 484–496. - PMC - PubMed
-
- Kumar N, Gahlawat A, Kumar RN, Singh YP, Modi G, Garg P, Drug repurposing for Alzheimer’s disease: in silico and in vitro investigation of FDA-approved drugs as acetylcholinesterase inhibitors, J. Biomol. Struct. Dyn 40 (2022) 2878–2892. - PubMed
-
- Van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Lecanemab in early Alzheimer’s disease, N. Engl. J. Med 388 (2023) 9–21. - PubMed
-
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N, NIA-AA research framework: toward a biological definition of Alzheimer’s disease, Alzheimers Dement. 14 (2018) 535–562. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

