Brain development and bioenergetic changes
- PMID: 38849103
- PMCID: PMC11495523
- DOI: 10.1016/j.nbd.2024.106550
Brain development and bioenergetic changes
Abstract
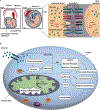
Bioenergetics describe the biochemical processes responsible for energy supply in organisms. When these changes become dysregulated in brain development, multiple neurodevelopmental diseases can occur, implicating bioenergetics as key regulators of neural development. Historically, the discovery of disease processes affecting individual stages of brain development has revealed critical roles that bioenergetics play in generating the nervous system. Bioenergetic-dependent neurodevelopmental disorders include neural tube closure defects, microcephaly, intellectual disability, autism spectrum disorders, epilepsy, mTORopathies, and oncogenic processes. Developmental timing and cell-type specificity of these changes determine the long-term effects of bioenergetic disease mechanisms on brain form and function. Here, we discuss key metabolic regulators of neural progenitor specification, neuronal differentiation (neurogenesis), and gliogenesis. In general, transitions between glycolysis and oxidative phosphorylation are regulated in early brain development and in oncogenesis, and reactive oxygen species (ROS) and mitochondrial maturity play key roles later in differentiation. We also discuss how bioenergetics interface with the developmental regulation of other key neural elements, including the cerebrospinal fluid brain environment. While questions remain about the interplay between bioenergetics and brain development, this review integrates the current state of known key intersections between these processes in health and disease.
Keywords: Bioenergetic pathways; Neural progenitors; Neurodevelopmental diseases.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflicts of interest.
Figures

Similar articles
-
Unraveling the Interplay Between Metabolism and Neurodevelopment in Health and Disease.CNS Neurosci Ther. 2025 May;31(5):e70427. doi: 10.1111/cns.70427. CNS Neurosci Ther. 2025. PMID: 40365712 Free PMC article. Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Aberrant cortical development is driven by impaired cell cycle and translational control in a DDX3X syndrome model.Elife. 2022 Jun 28;11:e78203. doi: 10.7554/eLife.78203. Elife. 2022. PMID: 35762573 Free PMC article.
-
In vivo models to study neurogenesis and associated neurodevelopmental disorders-Microcephaly and autism spectrum disorder.WIREs Mech Dis. 2023 Jul-Aug;15(4):e1603. doi: 10.1002/wsbm.1603. Epub 2023 Feb 8. WIREs Mech Dis. 2023. PMID: 36754084 Review.
-
Role of cell metabolism in the pathophysiology of brain size-associated neurodevelopmental disorders.Neurobiol Dis. 2024 Sep;199:106607. doi: 10.1016/j.nbd.2024.106607. Epub 2024 Jul 17. Neurobiol Dis. 2024. PMID: 39029564 Review.
Cited by
-
Adaptive protein synthesis in genetic models of copper deficiency and childhood neurodegeneration.Mol Biol Cell. 2025 Mar 1;36(3):ar33. doi: 10.1091/mbc.E24-11-0512. Epub 2025 Jan 29. Mol Biol Cell. 2025. PMID: 39878654 Free PMC article.
-
A primer on copper biology in the brain.Neurobiol Dis. 2025 Aug;212:106974. doi: 10.1016/j.nbd.2025.106974. Epub 2025 May 23. Neurobiol Dis. 2025. PMID: 40414313 Free PMC article. Review.
-
Adaptive protein synthesis in genetic models of copper deficiency and childhood neurodegeneration.bioRxiv [Preprint]. 2024 Nov 18:2024.09.09.612106. doi: 10.1101/2024.09.09.612106. bioRxiv. 2024. Update in: Mol Biol Cell. 2025 Mar 01;36(3):ar33. doi: 10.1091/mbc.E24-11-0512. PMID: 39314281 Free PMC article. Updated. Preprint.
-
Pre-Conception Maternal Obesity Confers Autism Spectrum Disorder-like Behaviors in Mice Offspring Through Neuroepigenetic Dysregulation.Cells. 2025 Aug 5;14(15):1201. doi: 10.3390/cells14151201. Cells. 2025. PMID: 40801633 Free PMC article.
References
-
- Aleck KA, Kaplan AM, Sherwood WG, Robinson BH, 1988. In utero central nervous system damage in pyruvate dehydrogenase deficiency. Arch. Neurol 45, 987–989. - PubMed
-
- Almeida A, Gonzalez-Buitrago JM, Bolanos JP, Medina JM, 1996. Fuel utilization by early newborn brain is preserved under congenital hypothyroidism in the rat. Pediatr. Res 40, 410–414. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

