Safety and efficacy of tofacitinib for the treatment of patients with juvenile idiopathic arthritis: preliminary results of an open-label, long-term extension study
- PMID: 38849152
- PMCID: PMC11503147
- DOI: 10.1136/ard-2023-225094
Safety and efficacy of tofacitinib for the treatment of patients with juvenile idiopathic arthritis: preliminary results of an open-label, long-term extension study
Abstract
Objectives: We report the safety, tolerability and efficacy of tofacitinib in patients with juvenile idiopathic arthritis (JIA) in an ongoing long-term extension (LTE) study.
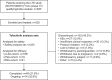
Methods: Patients (2-<18 years) with JIA who completed phase 1/3 index studies or discontinued for reasons excluding treatment-related serious adverse events (AEs) entered the LTE study and received tofacitinib 5 mg two times per day or equivalent weight-based doses. Safety outcomes included AEs, serious AEs and AEs of special interest. Efficacy outcomes included improvement since tofacitinib initiation per the JIA-American College of Rheumatology (ACR)70/90 criteria, JIA flare rate and disease activity measured by Juvenile Arthritis Disease Activity Score (JADAS)27, with inactive disease corresponding to JADAS ≤1.0.
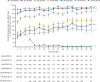
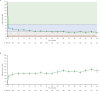
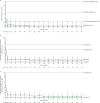
Results: Of 225 patients with JIA (median (range) duration of treatment, 41.6 (1-103) months), 201 (89.3%) had AEs; 34 (15.1%) had serious AEs. 10 patients developed serious infections; three had herpes zoster. Two patients newly developed uveitis. Among patients with polyarticular course JIA, JIA-ACR70/90 response rates were 60.0% (78 of 130) and 33.6% (47 of 140), respectively, at month 1, and generally improved over time. JIA flare events generally occurred in <5% of patients through to month 48. Observed mean (SE) JADAS27 was 22.0 (0.6) at baseline, 6.2 (0.7) at month 1 and 2.8 (0.5) at month 48, with inactive disease in 28.8% (36 of 125) of patients at month 1 and 46.8% (29 of 82) at month 48.
Conclusions: In this interim analysis of LTE study data in patients with JIA, safety findings were consistent with the known profile of tofacitinib, and efficacy was maintained up to month 48.
Trial registration number: NCT01500551.
Keywords: Antirheumatic Agents; Arthritis, Juvenile; Therapeutics.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ on behalf of EULAR.
Conflict of interest statement
Competing interests: HIB has received research grants from Bristol Myers Squibb, Novartis and Pfizer; is an employee of Cincinnati Children’s Hospital Medical Center; has received consulting fees or other remuneration from AbbVie, AstraZeneca/MedImmune, Bayer, Biocon, Boehringer Ingelheim, Bristol Myers Squibb, Cerecor, Eli Lilly, EMD Serono, Janssen, Novartis, Pfizer, Roche, R-Pharm and Sobi; and is a member of speaker bureaus for Novartis and Pfizer. JDA has received honorarium from Novartis. JFB has received research grants from AbbVie, Bristol Myers Squibb, Janssen, Pfizer and Roche. ALB is a member of speaker bureaus for AbbVie, Novartis and Roche. RC has received consulting fees or other remuneration from AbbVie, Bristol Myers Squibb, Eli Lilly, GSK, Novartis, Pfizer, Roche and Sanofi. GS has received consulting fees or other remuneration from Novartis and Sobi and research support from IpiNovyx. CN has received research grants from AbbVie, AstraZeneca, Bristol Myers Squibb, Eli Lilly, GSK, Pfizer and UCB. MV-DM is an employee of Clínica de Investigacion en Reumatologia y Obesidad, SC. HP, CC, CWh, KeK and SL are employees and stockholders of Pfizer. AW was an employee and stockholder of Pfizer at the time of this analysis. AM has received consulting fees or other remuneration from Aurinia, Bristol Myers Squibb, Eli Lilly, EMD Serono, Janssen and Pfizer. DL’s institution, the Cincinnati Children’s Hospital Medical Center, has received research grants from Bristol Myers Squibb, Janssen, Novartis, Pfizer, Roche and UBC; and has received consulting fees or other remuneration from AstraZeneca, Boehringer Ingelheim, GSK, Roche, Novartis, Pfizer, Takeda and UBC. DL is also a data safety and monitoring board member or chairperson for the National Institutes of Health and the Canadian Arthritis Society. NR has received honoraria for consultancies or speaker bureaus from Ablynx, AstraZeneca/MedImmune, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, EMD Serono, F Hoffmann-La Roche, GS, Janssen, MSD, Novartis, Pfizer, R-Pharm, Sanofi, Servier, Sinergie and Sobi. The IRCCS Istituto Giannina Gaslini, where NR works as a full-time public employee, has received contributions from Bristol Myers Squibb, Eli Lilly, F Hoffmann-La Roche, GS, Janssen, Novartis, Pfizer and Sobi; this funding has been reinvested for the research activities of the hospital in a fully independent manner, without any commitment with third parties. EA-A, GC, WDLP, LJ, ÖK, KaK, RR-C, JCRR, LW-W, JEW and CWo have declared no conflicts.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

