Mitochondrial ribosome biogenesis and redox sensing
- PMID: 38849194
- PMCID: PMC11452305
- DOI: 10.1002/2211-5463.13844
Mitochondrial ribosome biogenesis and redox sensing
Abstract
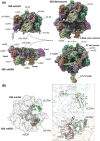
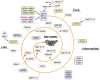
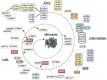
Mitoribosome biogenesis is a complex process involving RNA elements encoded in the mitochondrial genome and mitoribosomal proteins typically encoded in the nuclear genome. This process is orchestrated by extra-ribosomal proteins, nucleus-encoded assembly factors, which play roles across all assembly stages to coordinate ribosomal RNA processing and maturation with the sequential association of ribosomal proteins. Both biochemical studies and recent cryo-EM structures of mammalian mitoribosomes have provided insights into their assembly process. In this article, we will briefly outline the current understanding of mammalian mitoribosome biogenesis pathways and the factors involved. Special attention is devoted to the recent identification of iron-sulfur clusters as structural components of the mitoribosome and a small subunit assembly factor, the existence of redox-sensitive cysteines in mitoribosome proteins and assembly factors, and the role they may play as redox sensor units to regulate mitochondrial translation under stress.
Keywords: iron–sulfur cluster; mitochondrial disease; mitochondrial ribosome; mitochondrial translation; mitoribosome assembly; redox sensing.
© 2024 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

