ASXLs binding to the PHD2/3 fingers of MLL4 provides a mechanism for the recruitment of BAP1 to active enhancers
- PMID: 38849395
- PMCID: PMC11161652
- DOI: 10.1038/s41467-024-49391-x
ASXLs binding to the PHD2/3 fingers of MLL4 provides a mechanism for the recruitment of BAP1 to active enhancers
Abstract
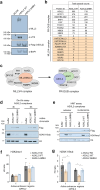
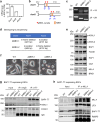
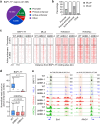
The human methyltransferase and transcriptional coactivator MLL4 and its paralog MLL3 are frequently mutated in cancer. MLL4 and MLL3 monomethylate histone H3K4 and contain a set of uncharacterized PHD fingers. Here, we report a novel function of the PHD2 and PHD3 (PHD2/3) fingers of MLL4 and MLL3 that bind to ASXL2, a component of the Polycomb repressive H2AK119 deubiquitinase (PR-DUB) complex. The structure of MLL4 PHD2/3 in complex with the MLL-binding helix (MBH) of ASXL2 and mutational analyses reveal the molecular mechanism which is conserved in homologous ASXL1 and ASXL3. The native interaction of the Trithorax MLL3/4 complexes with the PR-DUB complex in vivo depends solely on MBH of ASXL1/2, coupling the two histone modifying activities. ChIP-seq analysis in embryonic stem cells demonstrates that MBH of ASXL1/2 is required for the deubiquitinase BAP1 recruitment to MLL4-bound active enhancers. Our findings suggest an ASXL1/2-dependent functional link between the MLL3/4 and PR-DUB complexes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

