Impact of in vitro SARS-CoV-2 infection on breast cancer cells
- PMID: 38849411
- PMCID: PMC11161491
- DOI: 10.1038/s41598-024-63804-3
Impact of in vitro SARS-CoV-2 infection on breast cancer cells
Abstract
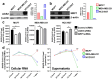
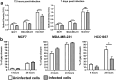
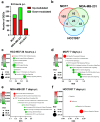
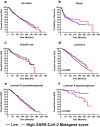
The pandemic of coronavirus disease 19 (COVID-19), caused by severe respiratory syndrome coronavirus 2 (SARS-CoV-2), had severe repercussions for breast cancer patients. Increasing evidence indicates that SARS-CoV-2 infection may directly impact breast cancer biology, but the effects of SARS-CoV-2 on breast tumor cells are still unknown. Here, we analyzed the molecular events occurring in the MCF7, MDA-MB-231 and HCC1937 breast cancer cell lines, representative of the luminal A, basal B/claudin-low and basal A subtypes, respectively, upon SARS-CoV-2 infection. Viral replication was monitored over time, and gene expression profiling was conducted. We found that MCF7 cells were the most permissive to viral replication. Treatment of MCF7 cells with Tamoxifen reduced the SARS-CoV-2 replication rate, suggesting an involvement of the estrogen receptor in sustaining virus replication in malignant cells. Interestingly, a metagene signature based on genes upregulated by SARS-CoV-2 infection in all three cell lines distinguished a subgroup of premenopausal luminal A breast cancer patients with a poor prognosis. As SARS-CoV-2 still spreads among the population, it is essential to understand the impact of SARS-CoV-2 infection on breast cancer, particularly in premenopausal patients diagnosed with the luminal A subtype, and to assess the long-term impact of COVID-19 on breast cancer outcomes.
Keywords: Breast cancer; Estrogen receptor; Luminal A breast cancer; SARS-CoV-2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The potential role of abnormal angiotensin-converting enzyme 2 expression correlated with immune infiltration after SARS-CoV-2 infection in the prognosis of breast cancer.Aging (Albany NY). 2021 Aug 19;13(17):20886-20895. doi: 10.18632/aging.203418. Epub 2021 Aug 19. Aging (Albany NY). 2021. PMID: 34413267 Free PMC article.
-
Raptor localization predicts prognosis and tamoxifen response in estrogen receptor-positive breast cancer.Breast Cancer Res Treat. 2018 Feb;168(1):17-27. doi: 10.1007/s10549-017-4508-x. Epub 2017 Nov 11. Breast Cancer Res Treat. 2018. PMID: 29128895 Free PMC article.
-
The signature of SARS-CoV-2-related genes predicts the immune therapeutic response and prognosis in breast cancer.BMC Med Genomics. 2024 Oct 31;17(1):260. doi: 10.1186/s12920-024-02032-0. BMC Med Genomics. 2024. PMID: 39482662 Free PMC article.
-
How SARS-CoV-2 (COVID-19) spreads within infected hosts - what we know so far.Emerg Top Life Sci. 2020 Dec 11;4(4):371-378. doi: 10.1042/ETLS20200165. Emerg Top Life Sci. 2020. PMID: 33269805 Free PMC article. Review.
-
Estrogen Receptor Modulators in Viral Infections Such as SARS-CoV-2: Therapeutic Consequences.Int J Mol Sci. 2021 Jun 18;22(12):6551. doi: 10.3390/ijms22126551. Int J Mol Sci. 2021. PMID: 34207220 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

