Stress-strain curve and elastic behavior of the fibrotic lung with usual interstitial pneumonia pattern during protective mechanical ventilation
- PMID: 38849437
- PMCID: PMC11161630
- DOI: 10.1038/s41598-024-63670-z
Stress-strain curve and elastic behavior of the fibrotic lung with usual interstitial pneumonia pattern during protective mechanical ventilation
Abstract
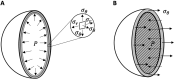
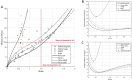
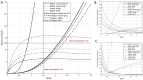
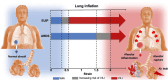
Patients with acute exacerbation of lung fibrosis with usual interstitial pneumonia (EUIP) pattern are at increased risk for ventilator-induced lung injury (VILI) and mortality when exposed to mechanical ventilation (MV). Yet, lack of a mechanical model describing UIP-lung deformation during MV represents a research gap. Aim of this study was to develop a constitutive mathematical model for UIP-lung deformation during lung protective MV based on the stress-strain behavior and the specific elastance of patients with EUIP as compared to that of acute respiratory distress syndrome (ARDS) and healthy lung. Partitioned lung and chest wall mechanics were assessed for patients with EUIP and primary ARDS (1:1 matched based on body mass index and PaO2/FiO2 ratio) during a PEEP trial performed within 24 h from intubation. Patient's stress-strain curve and the lung specific elastance were computed and compared with those of healthy lungs, derived from literature. Respiratory mechanics were used to fit a novel mathematical model of the lung describing mechanical-inflation-induced lung parenchyma deformation, differentiating the contributions of elastin and collagen, the main components of lung extracellular matrix. Five patients with EUIP and 5 matched with primary ARDS were included and analyzed. Global strain was not different at low PEEP between the groups. Overall specific elastance was significantly higher in EUIP as compared to ARDS (28.9 [22.8-33.2] cmH2O versus 11.4 [10.3-14.6] cmH2O, respectively). Compared to ARDS and healthy lung, the stress/strain curve of EUIP showed a steeper increase, crossing the VILI threshold stress risk for strain values greater than 0.55. The contribution of elastin was prevalent at lower strains, while the contribution of collagen was prevalent at large strains. The stress/strain curve for collagen showed an upward shift passing from ARDS and healthy lungs to EUIP lungs. During MV, patients with EUIP showed different respiratory mechanics, stress-strain curve and specific elastance as compared to ARDS patients and healthy subjects and may experience VILI even when protective MV is applied. According to our mathematical model of lung deformation during mechanical inflation, the elastic response of UIP-lung is peculiar and different from ARDS. Our data suggest that patients with EUIP experience VILI with ventilatory setting that are lung-protective for patients with ARDS.
Keywords: Acute respiratory distress syndrome; Acute respiratory failure; End-expiratory transpulmonary pressure; End-inspiratory transpulmonary pressure; Idiopathic pulmonary fibrosis; Interstitial lung disease; Invasive mechanical ventilation; Lung elastance; Lung fibrosis; Respiratory mechanics; Specific elastance; Strain; Stress; Transpulmonary pressure; Usual interstitial pneumonia; Ventilator-induced lung injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Physiological effects of lung-protective ventilation in patients with lung fibrosis and usual interstitial pneumonia pattern versus primary ARDS: a matched-control study.Crit Care. 2023 Oct 18;27(1):398. doi: 10.1186/s13054-023-04682-5. Crit Care. 2023. PMID: 37853480 Free PMC article.
-
Rethinking ARDS classification: oxygenation impairment fails to predict VILI risk.Intensive Care Med. 2025 Jan;51(1):62-71. doi: 10.1007/s00134-024-07712-0. Epub 2024 Dec 11. Intensive Care Med. 2025. PMID: 39661133 Free PMC article.
-
Inspiratory Effort and Respiratory Mechanics in Patients with Acute Exacerbation of Idiopathic Pulmonary fibrosis: A Preliminary Matched Control Study.Pulmonology. 2023 Nov-Dec;29(6):469-477. doi: 10.1016/j.pulmoe.2022.08.004. Epub 2022 Sep 28. Pulmonology. 2023. PMID: 36180352
-
How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients.Eur Respir J Suppl. 2003 Aug;42:15s-21s. doi: 10.1183/09031936.03.00420303. Eur Respir J Suppl. 2003. PMID: 12945996 Review.
-
The future of mechanical ventilation: lessons from the present and the past.Crit Care. 2017 Jul 12;21(1):183. doi: 10.1186/s13054-017-1750-x. Crit Care. 2017. PMID: 28701178 Free PMC article. Review.
Cited by
-
Quantitative CT-analysis of over aerated lung tissue and correlation with fibrosis extent in patients with idiopathic pulmonary fibrosis.Respir Res. 2024 Oct 5;25(1):359. doi: 10.1186/s12931-024-02970-4. Respir Res. 2024. PMID: 39369240 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

