Fabrication of heart tubes from iPSC derived cardiomyocytes and human fibrinogen by rotating mold technology
- PMID: 38849457
- PMCID: PMC11161509
- DOI: 10.1038/s41598-024-64022-7
Fabrication of heart tubes from iPSC derived cardiomyocytes and human fibrinogen by rotating mold technology
Abstract
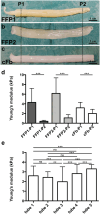
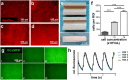
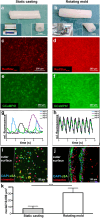
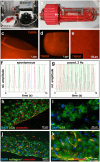
Due to its structural and functional complexity the heart imposes immense physical, physiological and electromechanical challenges on the engineering of a biological replacement. Therefore, to come closer to clinical translation, the development of a simpler biological assist device is requested. Here, we demonstrate the fabrication of tubular cardiac constructs with substantial dimensions of 6 cm in length and 11 mm in diameter by combining human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) and human foreskin fibroblast (hFFs) in human fibrin employing a rotating mold technology. By centrifugal forces employed in the process a cell-dense layer was generated enabling a timely functional coupling of iPSC-CMs demonstrated by a transgenic calcium sensor, rhythmic tissue contractions, and responsiveness to electrical pacing. Adjusting the degree of remodeling as a function of hFF-content and inhibition of fibrinolysis resulted in stable tissue integrity for up to 5 weeks. The rotating mold device developed in frame of this work enabled the production of tubes with clinically relevant dimensions of up to 10 cm in length and 22 mm in diameter which-in combination with advanced bioreactor technology for controlled production of functional iPSC-derivatives-paves the way towards the clinical translation of a biological cardiac assist device.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Maturation of human cardiomyocytes derived from induced pluripotent stem cells (iPSC-CMs) on polycaprolactone and polyurethane nanofibrous mats.Sci Rep. 2024 Jun 5;14(1):12975. doi: 10.1038/s41598-024-63905-z. Sci Rep. 2024. PMID: 38839879 Free PMC article.
-
Micropattern platform promotes extracellular matrix remodeling by human PSC-derived cardiac fibroblasts and enhances contractility of co-cultured cardiomyocytes.Physiol Rep. 2021 Oct;9(19):e15045. doi: 10.14814/phy2.15045. Physiol Rep. 2021. PMID: 34617673 Free PMC article.
-
Fabrication of Cardiac Constructs Using Bio-3D Printer.Methods Mol Biol. 2021;2320:53-63. doi: 10.1007/978-1-0716-1484-6_6. Methods Mol Biol. 2021. PMID: 34302647
-
Stem Cells and Their Cardiac Derivatives for Cardiac Tissue Engineering and Regenerative Medicine.Antioxid Redox Signal. 2021 Jul 20;35(3):143-162. doi: 10.1089/ars.2020.8193. Epub 2020 Nov 12. Antioxid Redox Signal. 2021. PMID: 32993354 Review.
-
Bioengineering methods for myocardial regeneration.Adv Drug Deliv Rev. 2016 Jan 15;96:195-202. doi: 10.1016/j.addr.2015.06.012. Epub 2015 Jul 4. Adv Drug Deliv Rev. 2016. PMID: 26150344 Free PMC article. Review.
Cited by
-
Bioprinting approaches in cardiac tissue engineering to reproduce blood-pumping heart function.iScience. 2024 Dec 20;28(1):111664. doi: 10.1016/j.isci.2024.111664. eCollection 2025 Jan 17. iScience. 2024. PMID: 39868032 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

