Synthetic retinoid-mediated preconditioning of cancer-associated fibroblasts and macrophages improves cancer response to immune checkpoint blockade
- PMID: 38849479
- PMCID: PMC11263587
- DOI: 10.1038/s41416-024-02734-3
Synthetic retinoid-mediated preconditioning of cancer-associated fibroblasts and macrophages improves cancer response to immune checkpoint blockade
Abstract
Background: The proliferation of cancer-associated fibroblasts (CAFs) hampers drug delivery and anti-tumor immunity, inducing tumor resistance to immune checkpoint blockade (ICB) therapy. However, it has remained a challenge to develop therapeutics that specifically target or modulate CAFs.
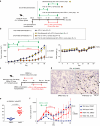
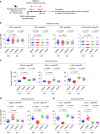
Methods: We investigated the involvement of Meflin+ cancer-restraining CAFs (rCAFs) in ICB efficacy in patients with clear cell renal cell carcinoma (ccRCC) and urothelial carcinoma (UC). We examined the effects of Am80 (a synthetic retinoid) administration on CAF phenotype, the tumor immune microenvironment, and ICB efficacy in cancer mouse models.
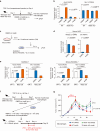
Results: High infiltration of Meflin+ CAFs correlated with ICB efficacy in patients with ccRCC and UC. Meflin+ CAF induction by Am80 administration improved ICB efficacy in the mouse models of cancer. Am80 exerted this effect when administered prior to, but not concomitant with, ICB therapy in wild-type but not Meflin-deficient mice. Am80-mediated induction of Meflin+ CAFs was associated with increases in antibody delivery and M1-like tumor-associated macrophage (TAM) infiltration. Finally, we showed the role of Chemerin produced from CAFs after Am80 administration in the induction of M1-like TAMs.
Conclusion: Our data suggested that Am80 administration prior to ICB therapy increases the number of Meflin+ rCAFs and ICB efficacy by inducing changes in TAM phenotype.
© 2024. The Author(s).
Conflict of interest statement
TM has financial interests in Denka Co., Ltd. The other authors declare no conflicts of interest.
Figures



References
-
- Helms E, Onate MK, Sherman MH. Fibroblast heterogeneity in the pancreatic tumor microenvironment. Cancer Discov. 2020;10:648–56. 10.1158/2159-8290.CD-19-1353. 10.1158/2159-8290.CD-19-1353 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 23ck0106779h0002/Japan Agency for Medical Research and Development (AMED)
- 23lk0221167h0001/Japan Agency for Medical Research and Development (AMED)
- 23lk0221172h0001/Japan Agency for Medical Research and Development (AMED)
- 22H02848/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 22K18390/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
LinkOut - more resources
Full Text Sources
Medical

