Anti-IL-5 Pathway Agents in Eosinophilic-Associated Disorders Across the Lifespan
- PMID: 38849701
- PMCID: PMC11196311
- DOI: 10.1007/s40265-024-02037-0
Anti-IL-5 Pathway Agents in Eosinophilic-Associated Disorders Across the Lifespan
Erratum in
-
Correction: Anti-IL-5 Pathway Agents in Eosinophilic-Associated Disorders Across the Lifespan.Drugs. 2025 Apr;85(4):599. doi: 10.1007/s40265-025-02149-1. Epub 2025 Feb 22. Drugs. 2025. PMID: 39985742 Free PMC article. No abstract available.
Abstract
Monoclonal antibodies targeting interleukin (IL)-5 pathways have revolutionized the treatment expectations for eosinophilic-associated conditions, particularly in patients with respiratory involvement. Mepolizumab (IL-5 antagonist monoclonal antibody), benralizumab (IL-5 receptor blocker monoclonal antibody), and reslizumab (IL-5 antagonist monoclonal antibody) have collectively contributed to the overall improvement of the disease burden in various conditions. Eosinophilic asthma currently boasts the most robust evidence across all age groups: all three biologics are approved for adults (aged ≥18 years); mepolizumab is approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) also in children (aged ≥ 6 years), while bernalizumab was recently approved by the FDA for patients aged ≥6 years in the USA. In chronic rhinosinusitis with nasal polyps, subcutaneous mepolizumab is the only anti-IL-5 therapy approved so far and can be used in adult patients (aged ≥18 years). For eosinophilic esophagitis, conflicting evidence surrounds both mepolizumab, reslizumab, and benralizumab, leading to non-approval of these agents by the FDA/EMA. Recently, mepolizumab was approved for eosinophilic granulomatosis with polyangiitis patients aged ≥6 years or older and for hypereosinophilic syndrome adult patients. A phase III trial proving noninferiority of benralizumab versus mepolizumab in eosinophilic granulomatosis with polyangiitis has been recently published, while evidence on reslizumab is scant. Overall, current evidence on anti-IL-5 biologics for eosinophilic-associated disorders is mostly focused on adults, whereas data for individuals aged under 18 years and over 65 years are scarce, resulting in a lack of evidence, particularly regarding efficacy, for the use of anti-IL-5 agents in these specific patient populations. This review addresses high-quality evidence from randomized controlled trials and real-world post-marketing studies regarding the use of anti-IL-5 therapies for eosinophilic-associated disorders across all age groups, spanning childhood, adulthood, and older age.
© 2024. The Author(s).
Conflict of interest statement
Alvise Berti received funding from GSK (advisory boards, speaker fees). Overall, the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


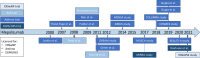


Similar articles
-
Evidence for the efficacy and safety of anti-interleukin-5 treatment in the management of refractory eosinophilic asthma.Ther Adv Respir Dis. 2015 Aug;9(4):135-45. doi: 10.1177/1753465815581279. Epub 2015 Apr 21. Ther Adv Respir Dis. 2015. PMID: 25900924 Review.
-
The spectrum of therapeutic activity of mepolizumab.Expert Rev Clin Immunol. 2019 Sep;15(9):959-967. doi: 10.1080/1744666X.2019.1656065. Epub 2019 Aug 29. Expert Rev Clin Immunol. 2019. PMID: 31424304 Review.
-
Mepolizumab: 240563, anti-IL-5 monoclonal antibody - GlaxoSmithKline, anti-interleukin-5 monoclonal antibody - GlaxoSmithKline, SB 240563.Drugs R D. 2008;9(2):125-30. doi: 10.2165/00126839-200809020-00006. Drugs R D. 2008. PMID: 18298130 Review.
-
Severe eosinophilic asthma: from the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab.Drug Des Devel Ther. 2017 Oct 30;11:3137-3144. doi: 10.2147/DDDT.S150656. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 29133975 Free PMC article. Review.
-
Mepolizumab: First Global Approval.Drugs. 2015 Dec;75(18):2163-9. doi: 10.1007/s40265-015-0513-8. Drugs. 2015. PMID: 26603873 Review.
Cited by
-
Causality between various cytokines and asthma: a bidirectional two-sample Mendelian randomization analysis.Front Med (Lausanne). 2024 Aug 8;11:1447673. doi: 10.3389/fmed.2024.1447673. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39175819 Free PMC article.
-
Eosinophilia with STAT5BN642H Mutation: A Heterogeneous Entity with Overlapping Morphological Features and Poor Outcome.Turk J Haematol. 2024 Dec 2;41(4):275-278. doi: 10.4274/tjh.galenos.2024.2024.0204. Epub 2024 Aug 29. Turk J Haematol. 2024. PMID: 39206794 Free PMC article. No abstract available.
-
Sinonasal Outcomes Obtained after 2 Years of Treatment with Benralizumab in Patients with Severe Eosinophilic Asthma and CRSwNP: A "Real-Life" Observational Study.J Pers Med. 2024 Sep 23;14(9):1014. doi: 10.3390/jpm14091014. J Pers Med. 2024. PMID: 39338268 Free PMC article.
-
Hypereosinophilia: clinical and therapeutic approach in 2025.Curr Opin Allergy Clin Immunol. 2025 Aug 1;25(4):258-268. doi: 10.1097/ACI.0000000000001078. Epub 2025 May 21. Curr Opin Allergy Clin Immunol. 2025. PMID: 40396537 Free PMC article. Review.
-
Clinical and Pathophysiological Tangles Between Allergy and Autoimmunity: Deconstructing an Old Dichotomic Paradigm.Clin Rev Allergy Immunol. 2025 Feb 11;68(1):13. doi: 10.1007/s12016-024-09020-3. Clin Rev Allergy Immunol. 2025. PMID: 39932658 Free PMC article. Review.
References
-
- Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–9. 10.1093/intimm/dxp102. - PubMed
-
- Keating GM. Mepolizumab: first global approval. Drugs. 2015;75(18):2163–9. 10.1007/s40265-015-0513-8. - PubMed
-
- Deeks ED, Brusselle G. Reslizumab in eosinophilic asthma: a review. Drugs. 2017;77(7):777–84. 10.1007/s40265-017-0740-2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

