Mechanism and bioinformatics analysis of the effect of berberine-enhanced fluconazole against drug-resistant Candida albicans
- PMID: 38849761
- PMCID: PMC11157861
- DOI: 10.1186/s12866-024-03334-0
Mechanism and bioinformatics analysis of the effect of berberine-enhanced fluconazole against drug-resistant Candida albicans
Abstract
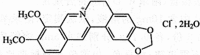
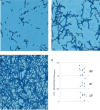
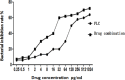
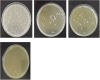
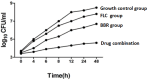
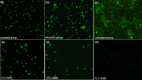
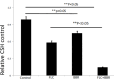
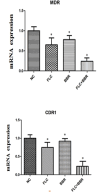
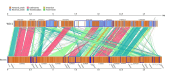
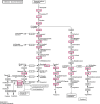
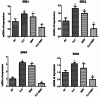
Biofilms produced by Candida albicans present a challenge in treatment with antifungal drug. Enhancing the sensitivity to fluconazole (FLC) is a reasonable method for treating FLC-resistant species. Moreover, several lines of evidence have demonstrated that berberine (BBR) can have antimicrobial effects. The aim of this study was to clarify the underlying mechanism of these effects. We conducted a comparative study of the inhibition of FLC-resistant strain growth by FLC treatment alone, BBR treatment alone, and the synergistic effect of combined FLC and BBR treatment. Twenty-four isolated strains showed distinct biofilm formation capabilities. The antifungal effect of combined FLC and BBR treatment in terms of the growth and biofilm formation of Candida albicans species was determined via checkerboard, time-kill, and fluorescence microscopy assays. The synergistic effect of BBR and FLC downregulated the expression of the efflux pump genes CDR1 and MDR, the hyphal gene HWP1, and the adhesion gene ALS3; however, the gene expression of the transcriptional repressor TUP1 was upregulated following treatment with this drug combination. Furthermore, the addition of BBR led to a marked reduction in cell surface hydrophobicity. To identify resistance-related genes and virulence factors through genome-wide sequencing analysis, we investigated the inhibition of related resistance gene expression by the combination of BBR and FLC, as well as the associated signaling pathways and metabolic pathways. The KEGG metabolic map showed that the metabolic genes in this strain are mainly involved in amino acid and carbon metabolism. The metabolic pathway map showed that several ergosterol (ERG) genes were involved in the synthesis of cell membrane sterols, which may be related to drug resistance. In this study, BBR + FLC combination treatment upregulated the expression of the ERG1, ERG3, ERG4, ERG5, ERG24, and ERG25 genes and downregulated the expression of the ERG6 and ERG9 genes compared with fluconazole treatment alone (p < 0.05).
Keywords: Candida albicans; Berberine; Biofilm; Ergosterol; Fluconazole.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Inhibitory effects of berberine on fungal growth, biofilm formation, virulence, and drug resistance as an antifungal drug and adjuvant with prospects for future applications.World J Microbiol Biotechnol. 2024 Dec 18;41(1):5. doi: 10.1007/s11274-024-04223-4. World J Microbiol Biotechnol. 2024. PMID: 39690297 Review.
-
Fluconazole assists berberine to kill fluconazole-resistant Candida albicans.Antimicrob Agents Chemother. 2013 Dec;57(12):6016-27. doi: 10.1128/AAC.00499-13. Epub 2013 Sep 23. Antimicrob Agents Chemother. 2013. PMID: 24060867 Free PMC article.
-
Requirement for Ergosterol in Berberine Tolerance Underlies Synergism of Fluconazole and Berberine against Fluconazole-Resistant Candida albicans Isolates.Front Cell Infect Microbiol. 2017 Nov 29;7:491. doi: 10.3389/fcimb.2017.00491. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29238700 Free PMC article.
-
Mechanism of berberine-mediated fluconazole-susceptibility enhancement in clinical fluconazole-resistant Candida tropicalis isolates.Biomed Pharmacother. 2017 Sep;93:709-712. doi: 10.1016/j.biopha.2017.06.106. Epub 2017 Jul 9. Biomed Pharmacother. 2017. PMID: 28700974
-
Proteomic analysis reveals a synergistic mechanism of fluconazole and berberine against fluconazole-resistant Candida albicans: endogenous ROS augmentation.J Proteome Res. 2009 Nov;8(11):5296-304. doi: 10.1021/pr9005074. J Proteome Res. 2009. PMID: 19754040
Cited by
-
Bioactive Plant Compounds as Alternatives Against Antifungal Resistance in the Candida Strains.Pharmaceutics. 2025 May 23;17(6):687. doi: 10.3390/pharmaceutics17060687. Pharmaceutics. 2025. PMID: 40574000 Free PMC article. Review.
-
Inhibitory effects of berberine on fungal growth, biofilm formation, virulence, and drug resistance as an antifungal drug and adjuvant with prospects for future applications.World J Microbiol Biotechnol. 2024 Dec 18;41(1):5. doi: 10.1007/s11274-024-04223-4. World J Microbiol Biotechnol. 2024. PMID: 39690297 Review.
-
Innovative antifungal strategies to combat drug-resistant Candida auris: recent advances and clinical implications.Front Cell Infect Microbiol. 2025 Jul 31;15:1641373. doi: 10.3389/fcimb.2025.1641373. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40822589 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

