125I seed brachytherapy for non-central pelvic recurrence of cervical cancer after external beam radiotherapy
- PMID: 38849839
- PMCID: PMC11162001
- DOI: 10.1186/s13014-024-02454-1
125I seed brachytherapy for non-central pelvic recurrence of cervical cancer after external beam radiotherapy
Abstract
Objective: To investigate the efficacy of 125I seed brachytherapy for non-central pelvic recurrence of cervical cancer after external beam radiotherapy, and to analyze the clinical influential factors.
Methods: Between June 2015 and April 2022, 32 patients with 41 lesions were treated with 125I seed brachytherapy. The seeds were implanted under the guidance of CT and/or 3D-printed template images at a median dose of 100 Gy (range, 80-120 Gy), and the local control rate (LCR) and survival rates were calculated. We used multivariate logistic regression to identify prognosis predictors, and receiver operating characteristic (ROC) curve analysis to determine the optimal cut-off values.
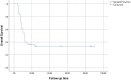
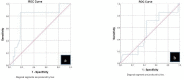
Results: The median follow-up was 48.52 months (range, 4-86 months), and the 6-, 12-, and 24-month LCR was 88.0%, 63.2%, and 42.1%, respectively. The 1- and 2-year survival rates were 36% and 33%, respectively, and the median survival time was 13.26 months. No significant adverse events occurred. Multivariate regression analysis showed that tumor diameter, tumor stage, and LCR were independent factors influencing survival. ROC curve analysis showed that the area under the curve for tumor diameter and D90 were 0.765 and 0.542, respectively, with cut-off values of 5.3 cm and 108.5 Gy.
Conclusions: The present findings indicate that 125I seed brachytherapy is feasible for treating non-central pelvic recurrence of cervical cancer after external beam radiotherapy. Further, tumor diameter < 5.3 cm and immediate postoperative D90 > 108.5 Gy were associated with better efficacy.
Keywords: Brachytherapy; External Beam Radiotherapy; Locoregional; Non-central pelvic recurrence of Cervical Cancer; Pelvic recurrence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Efficacy and dosimetry analysis of image-guided radioactive ¹²⁵I seed implantation as salvage treatment for pelvic recurrent cervical cancer after external beam radiotherapy.J Gynecol Oncol. 2019 Jan;30(1):e9. doi: 10.3802/jgo.2019.30.e9. Epub 2018 Oct 30. J Gynecol Oncol. 2019. PMID: 30479093 Free PMC article.
-
Transperineal low-dose rate iridium-192 interstitial brachytherapy in cervical carcinoma stage IIB.Strahlenther Onkol. 2001 Oct;177(10):517-24. doi: 10.1007/pl00002362. Strahlenther Onkol. 2001. PMID: 11680016
-
High-dose-rate versus low-dose-rate brachytherapy in the treatment of cervical cancer: analysis of tumor recurrence--the University of Wisconsin experience.Int J Radiat Oncol Biol Phys. 1999 Dec 1;45(5):1267-74. doi: 10.1016/s0360-3016(99)00262-x. Int J Radiat Oncol Biol Phys. 1999. PMID: 10613322
-
Efficacy and Safety of 125I Seed Implantation in the Treatment of Pelvic Recurrent Cervical Cancer Following Radiotherapy: A Single-Arm Meta-Analysis of Chinese Patients.Cancer Rep (Hoboken). 2024 Aug;7(8):e2147. doi: 10.1002/cnr2.2147. Cancer Rep (Hoboken). 2024. PMID: 39158182 Free PMC article. Review.
-
An audit of the treatment of carcinoma of the uterine cervix using external beam radiotherapy and a single line source brachytherapy technique.Br J Radiol. 1997 Dec;70(840):1259-69. doi: 10.1259/bjr.70.840.9505845. Br J Radiol. 1997. PMID: 9505845 Review.
Cited by
-
3D-Printed Devices in Interventional Radiotherapy (Brachytherapy) Applications: A Literature Review.J Pers Med. 2025 Jun 19;15(6):262. doi: 10.3390/jpm15060262. J Pers Med. 2025. PMID: 40559124 Free PMC article. Review.
-
125I inhibits the progression of cervical cancer by upregulating the HSF1/PU.1/SYK signaling pathway and consequently enhancing the apoptotic response mediated by ROS/USP7/P53.Sci Rep. 2025 May 21;15(1):17690. doi: 10.1038/s41598-025-99214-2. Sci Rep. 2025. PMID: 40399470 Free PMC article.
References
-
- Krishnansu S, Tewari MW, Sill RT, Penson et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240).The Lancet,2017,390(10103):1654–63. 10.1016/S0140-6736(17)31607-0. Epub 2017 Jul 27. - PMC - PubMed

