Lomustine with or without reirradiation for first progression of glioblastoma, LEGATO, EORTC-2227-BTG: study protocol for a randomized phase III study
- PMID: 38849943
- PMCID: PMC11157762
- DOI: 10.1186/s13063-024-08213-7
Lomustine with or without reirradiation for first progression of glioblastoma, LEGATO, EORTC-2227-BTG: study protocol for a randomized phase III study
Abstract
Background: Chemotherapy with lomustine is widely considered as standard treatment option for progressive glioblastoma. The value of adding radiotherapy to second-line chemotherapy is not known.
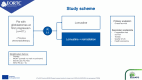
Methods: EORTC-2227-BTG (LEGATO, NCT05904119) is an investigator-initiated, pragmatic (PRECIS-2 score: 34 out of 45), randomized, multicenter phase III trial in patients with first progression of glioblastoma. A total of 411 patients will be randomized in a 1:1 ratio to lomustine (110 mg/m2 every 6 weeks) or lomustine (110 mg/m2 every 6weeks) plus radiotherapy (35 Gy in 10 fractions). Main eligibility criteria include histologic confirmation of glioblastoma, isocitrate dehydrogenase gene (IDH) wild-type per WHO 2021 classification, first progression at least 6 months after the end of prior radiotherapy, radiologically measurable disease according to RANO criteria with a maximum tumor diameter of 5 cm, and WHO performance status of 0-2. The primary efficacy endpoint is overall survival (OS) and secondary endpoints include progression-free survival, response rate, neurocognitive function, health-related quality of life, and health economic parameters. LEGATO is funded by the European Union's Horizon Europe Research program, was activated in March 2024 and will enroll patients in 43 sites in 11 countries across Europe with study completion projected in 2028.
Discussion: EORTC-2227-BTG (LEGATO) is a publicly funded pragmatic phase III trial designed to clarify the efficacy of adding reirradiation to chemotherapy with lomustine for the treatment of patients with first progression of glioblastoma.
Trial registration: ClinicalTrials.gov NCT05904119. Registered before start of inclusion, 23 May 2023.
Keywords: Glioblastoma; LEGATO; Lomustine; Progression; Randomized controlled trial; Reirradiation.
© 2024. The Author(s).
Conflict of interest statement
MP has received honoraria for lectures, consultation, or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals, Janssen, Servier, Miltenyi, Böhringer-Ingelheim, Telix, and Medscape.
JG has received honoraria for lectures, consultation, or advisory board participation from the following for-profit companies: Zeiss and Saegen.
JK: the author declares that he has no competing interests.
TK: the author declares that he has no competing interests.
FS: Honoraria from Illumina and co-founder and shareholder of Heidelberg Epignostix GmbH
TG, BF, and CQ (EORTC): the authors declare that they have no competing interests.
TGO: the author declares that he has no competing interests.
ELR has received research grants from Bristol Meyers Squibb (BMS), and honoraria for lectures or advisory board participation or consulting from Bayer, Janssen, Leo Pharma, Pierre Fabre, Roche, Seattle Genetics, and Servier.
MW has received research grants from Novartis, Quercis, and Versameb and honoraria for lectures or advisory board participation or consulting from Anheart, Bayer, Curevac, Medac, Neurosense, Novartis, Novocure, Orbus, Pfizer, Philogen, Roche, and Servier.
Figures
References
-
- Tsien CI, Pugh SL, Dicker AP, Raizer JJ, Matuszak MM, Lallana EC, et al. NRG Oncology/RTOG1205: a randomized phase II trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J Clin Oncol. 2023;41(6):1285–95. doi: 10.1200/JCO.22.00164. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical