Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer's disease: a randomized, controlled clinical trial
- PMID: 38849944
- PMCID: PMC11157928
- DOI: 10.1186/s13195-024-01482-z
Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer's disease: a randomized, controlled clinical trial
Abstract
Background: Evidence links lifestyle factors with Alzheimer's disease (AD). We report the first randomized, controlled clinical trial to determine if intensive lifestyle changes may beneficially affect the progression of mild cognitive impairment (MCI) or early dementia due to AD.
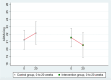
Methods: A 1:1 multicenter randomized controlled phase 2 trial, ages 45-90 with MCI or early dementia due to AD and a Montreal Cognitive Assessment (MoCA) score of 18 or higher. The primary outcome measures were changes in cognition and function tests: Clinical Global Impression of Change (CGIC), Alzheimer's Disease Assessment Scale (ADAS-Cog), Clinical Dementia Rating-Sum of Boxes (CDR-SB), and Clinical Dementia Rating Global (CDR-G) after 20 weeks of an intensive multidomain lifestyle intervention compared to a wait-list usual care control group. ADAS-Cog, CDR-SB, and CDR-Global scales were compared using a Mann-Whitney-Wilcoxon rank-sum test, and CGIC was compared using Fisher's exact test. Secondary outcomes included plasma Aβ42/40 ratio, other biomarkers, and correlating lifestyle with the degree of change in these measures.
Results: Fifty-one AD patients enrolled, mean age 73.5. No significant differences in any measures at baseline. Only two patients withdrew. All patients had plasma Aβ42/40 ratios <0.0672 at baseline, strongly supporting AD diagnosis. After 20 weeks, significant between-group differences in the CGIC (p= 0.001), CDR-SB (p= 0.032), and CDR Global (p= 0.037) tests and borderline significance in the ADAS-Cog test (p= 0.053). CGIC, CDR Global, and ADAS-Cog showed improvement in cognition and function and CDR-SB showed significantly less progression, compared to the control group which worsened in all four measures. Aβ42/40 ratio increased in the intervention group and decreased in the control group (p = 0.003). There was a significant correlation between lifestyle and both cognitive function and the plasma Aβ42/40 ratio. The microbiome improved only in the intervention group (p <0.0001).
Conclusions: Comprehensive lifestyle changes may significantly improve cognition and function after 20 weeks in many patients with MCI or early dementia due to AD.
Trial registration: Approved by Western Institutional Review Board on 12/31/2017 (#20172897) and by Institutional Review Boards of all sites. This study was registered retrospectively with clinicaltrials.gov on October 8, 2020 (NCT04606420, ID: 20172897).
Keywords: Alzheimer’s; Diet; Exercise; Lifestyle medicine; Nutrition; Social support; Stress management.
© 2024. The Author(s).
Conflict of interest statement
MK is one of the Editors-in-Chief of this journal and has no relevant competing interests and recused herself from the review process. RKD is an inventor on key patents in the field of metabolomics and holds equity in Metabolon, a biotech company in North Carolina. In addition, she holds patents licensed to Chymia LLC and PsyProtix with royalties and ownership. DO and AO have consulted for Sharecare and have received book royalties and lecture honoraria and, with CK, have received equity in Ornish Lifestyle Medicine. RK is a scientific advisory board member and consultant for BiomeSense, Inc., has equity and receives income. He is a scientific advisory board member and has equity in GenCirq. He is a consultant and scientific advisory board member for DayTwo, and receives income. He has equity in and acts as a consultant for Cybele. He is a co-founder of Biota, Inc., and has equity. He is a cofounder of Micronoma, and has equity and is a scientific advisory board member. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. DM is a consultant for BiomeSense. RT is a co-founder and equity holder in Hyperion Rx, which produces the flashing-light glasses at a theta frequency of 7.83 Hz used as an optional aid to meditation. The rest of the authors declare that they have no competing interests.
Figures
References
-
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. - DOI - PMC - PubMed
-
- Ornish D, Ornish A. UnDo It. New York: Ballantine Books; 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical