Impact of cemiplimab treatment duration on clinical outcomes in advanced cutaneous squamous cell carcinoma
- PMID: 38850335
- PMCID: PMC11162402
- DOI: 10.1007/s00262-024-03728-z
Impact of cemiplimab treatment duration on clinical outcomes in advanced cutaneous squamous cell carcinoma
Abstract
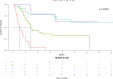
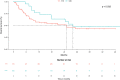
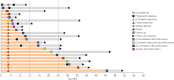
Treatment duration with checkpoint inhibitors must be optimized to prevent unjustified toxicity, but evidence for the management of cutaneous squamous cell carcinoma is lacking. A retrospective study was performed to evaluate the survival of patients with cutaneous squamous cell carcinoma (CSCC) who discontinued cemiplimab due to different causes and without progression. Among 95 patients with CSCC who received cemiplimab, 22 (23%) patients discontinued immunotherapy due to causes other than progression, such as comorbidities, toxicity, complete response or lack of compliance (group that discontinued before censoring [DBC]), then 73 patients had standard treatment scheduled (STS). The overall survival was 25.2 months (95% CI: 8.9-29.4) in STS group and 28.3 months (95% CI: 12.7-28.3) in the DBC group; deaths for all causes were 11/22 (50%) in the DBC group and 34/73 (46.6%) in the STS group (p = 0.32). 10/22 (45.4%) subjects died due to CSCC in the DBC after discontinuation and 34/73 (46.6%) in the STS group, and the difference between groups was not significant (p = 0.230). Duration of treatment was significantly lower in subjects with stable disease versus those with complete or partial response (16.9, 30.6 and 34.9 months, respectively; p = 0.004). Among the 22 STS patients, 12 received cemiplimab for less than 12 months (10 [83%] died) and 10 for at least 12 months (1 [10%] died). Our observation, finding no outcome difference between DBC and STS groups, suggests that ICI treatment after one year might expose patients to further treatment related events without efficacy advantages.
Keywords: Anti-PD1; Cemiplimab; Cutaneous squamous cell carcinoma; Duration of response; Immune checkpoint inhibitors; Immunotherapy.
© 2024. The Author(s).
Conflict of interest statement
PAA has/had a consultant/advisory role for Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, iTeos, Medicenna, Bio-Al Health. He also received research funding from Bristol Myers Squibb, Roche-Genentech, Pfizer, Sanofi. AC received grant consultancies from BMS, Astrazeneca, Roche and MSD. He also received speaker’s fee from Astrazeneca, Novartis and Eisai.
Figures


References
-
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345–1356. 10.1056/NEJMoa1709684 10.1056/NEJMoa1709684 - DOI - PMC - PubMed
-
- Marron TU, Ryan AE, Reddy SM, Kaczanowska S, Younis RH, Thakkar D, Zhang J, Bartkowiak T, Howard R, Anderson KG, Olson D, Naqash AR, Patel RB, Sachdev E, Rodriguez-Ruiz ME, Sheffer M, Church S, Fuhrman C, Overacre-Delgoffe A, Nguyen R, Florou V, Thaxton JE, Aggen DH, Guerriero JL (2021) Considerations for treatment duration in responders to immune checkpoint inhibitors. J Immunother Cancer 9:e001901. 10.1136/jitc-2020-001901 10.1136/jitc-2020-001901 - DOI - PMC - PubMed
-
- Mulder EEAP, de Joode K, Litière S, Ten Tije AJ, Suijkerbuijk KPM, Boers-Sonderen MJ, Hospers GAP, de Groot JWB, van den Eertwegh AJM, Aarts MJB, Piersma D, van Rijn RS, Kapiteijn E, Vreugdenhil G, van den Berkmortel FWPJ, Hoop EO, Franken MG, Ryll B, Rutkowski P, Sleijfer S, Haanen JBAG, van der Veldt AAM (2021) Early discontinuation of PD-1 blockade upon achieving a complete or partial response in patients with advanced melanoma: the multicentre prospective safe stop trial. BMC Cancer 21:1–9. 10.1186/s12885-021-08018-w 10.1186/s12885-021-08018-w - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

