More than the clock: distinct regulation of muscle function and metabolism by PER2 and RORα
- PMID: 38850551
- PMCID: PMC11607892
- DOI: 10.1113/JP285585
More than the clock: distinct regulation of muscle function and metabolism by PER2 and RORα
Abstract
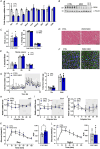
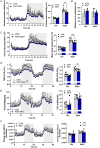
Circadian rhythms, governed by the dominant central clock, in addition to various peripheral clocks, regulate almost all biological processes, including sleep-wake cycles, hormone secretion and metabolism. In certain contexts, the regulation and function of the peripheral oscillations can be decoupled from the central clock. However, the specific mechanisms underlying muscle-intrinsic clock-dependent modulation of muscle function and metabolism remain unclear. We investigated the outcome of perturbations of the primary and secondary feedback loops of the molecular clock in skeletal muscle by specific gene ablation of Period circadian regulator 2 (Per2) and RAR-related orphan receptor alpha (Rorα), respectively. In both models, a dampening of core clock gene oscillation was observed, while the phase was preserved. Moreover, both loops seem to be involved in the homeostasis of amine groups. Highly divergent outcomes were seen for overall muscle gene expression, primarily affecting circadian rhythmicity in the PER2 knockouts and non-oscillating genes in the RORα knockouts, leading to distinct outcomes in terms of metabolome and phenotype. These results highlight the entanglement of the molecular clock and muscle plasticity and allude to specific functions of different clock components, i.e. the primary and secondary feedback loops, in this context. The reciprocal interaction between muscle contractility and circadian clocks might therefore be instrumental to determining a finely tuned adaptation of muscle tissue to perturbations in health and disease. KEY POINTS: Specific perturbations of the primary and secondary feedback loop of the molecular clock result in specific outcomes on muscle metabolism and function. Ablation of Per2 (primary loop) or Rorα (secondary loop) blunts the amplitude of core clock genes, in absence of a shift in phase. Perturbation of the primary feedback loop by deletion of PER2 primarily affects muscle gene oscillation. Knockout of RORα and the ensuing modulation of the secondary loop results in the aberrant expression of a large number of non-clock genes and proteins. The deletion of PER2 and RORα affects muscle metabolism and contractile function in a circadian manner, highlighting the central role of the molecular clock in modulating muscle plasticity.
Keywords: PER2; RORα; circadian rhythm; exercise; metabolism; molecular clock; skeletal muscle; transcriptional regulation.
© 2024 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
None declared.
Figures










References
-
- Ahrné, E. , Glatter, T. , Viganò, C. , von Schubert, C. , Nigg, E. A. , & Schmidt, A. (2016). Evaluation and improvement of quantification accuracy in isobaric mass tag‐based protein quantification experiments. Journal of Proteome Research, 15(8), 2537–2547. - PubMed
-
- Andrews, J. L. , Zhang, X. , McCarthy, J. J. , McDearmon, E. L. , Hornberger, T. A. , Russell, B. , Campbell, K. S. , Arbogast, S. , Reid, M. B. , Walker, J. R. , Hogenesch, J. B. , Takahashi, J. S. , & Esser, K. A. (2010). CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proceedings of the National Academy of Sciences of the United States of America, 107(44), 19090–19095 - PMC - PubMed
-
- Bae, K. , Jin, X. , Maywood, E. S. , Hastings, M. H. , Reppert, S. M. , & Weaver, D. R. (2001). Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron, 30(2), 525–536 - PubMed
-
- Bae, K. , Lee, K. , Seo, Y. , Lee, H. , Kim, D. , & Choi, I. (2006). Differential effects of two period genes on the physiology and proteomic profiles of mouse anterior tibialis muscles. Molecules and Cells, 22(3), 275–284. - PubMed
-
- Bass, J. , & Lazar, M. A. (2016). Circadian time signatures of fitness and disease. Science, 354(6315), 994–999. - PubMed
MeSH terms
Substances
Grants and funding
- CRSII5_209252/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- Swiss Society for Research on Muscle Diseases
- Novartis Stiftung für Medizinisch-Biologische Forschung
- 310030_184832/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- Biozentrum PhD Fellowship Program
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
