Animal models of immune-mediated demyelinating polyneuropathies
- PMID: 38850571
- PMCID: PMC11215812
- DOI: 10.1080/08916934.2024.2361745
Animal models of immune-mediated demyelinating polyneuropathies
Abstract
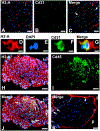
Immune-mediated demyelinating polyneuropathies (IMDPs) are rare disorders in which dysregulated adaptive immune responses cause peripheral nerve demyelinating inflammation and axonal injury in susceptible individuals. Despite significant advances in understanding IMDP pathogenesis guided by patient data and representative mammalian models, specific therapies are lacking. Significant knowledge gaps in IMDP pathogenesis still exist, e.g. precise antigen(s) and mechanisms that initially trigger immune system activation and identification of large population disease susceptibility factors. The initial directional cues for antigen-specific effector or autoreactive leukocyte trafficking into peripheral nerves are also unknown. An overview of current animal models, with emphasis on the experimental autoimmune neuritis and spontaneous autoimmune peripheral polyneuropathy models, is provided. Insights on the initial directional cues for peripheral nerve tissue specific autoimmunity using a novel Major Histocompatibility Complex class II conditional knockout mouse strain are also discussed, suggesting an essential research tool to study cell- and time-dependent adaptive immunity in autoimmune diseases.
Keywords: Acute inflammatory demyelinating polyradiculoneuropathy; animal models; blood-nerve barrier; chronic inflammatory demyelinating polyradiculoneuropathy; demyelinating neuritis; experimental autoimmune neuritis; major histocompatibility complex; mice; pathogenesis; spontaneous autoimmune peripheral polyneuropathy.
Figures

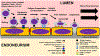

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
