Preventive effect of prenatal maternal oral probiotic supplementation on neonatal jaundice (POPS Study): A protocol for the randomised double-blind placebo-controlled clinical trial
- PMID: 38851232
- PMCID: PMC11163667
- DOI: 10.1136/bmjopen-2023-083641
Preventive effect of prenatal maternal oral probiotic supplementation on neonatal jaundice (POPS Study): A protocol for the randomised double-blind placebo-controlled clinical trial
Abstract
Introduction: Neonatal jaundice is a common and life-threatening health problem in neonates due to overaccumulation of circulating unconjugated bilirubin. Gut flora has a potential influence on bilirubin metabolism. The infant gut microbiome is commonly copied from the maternal gut. During pregnancy, due to changes in dietary habits, hormones and body weight, maternal gut dysbiosis is common, which can be stabilised by probiotics supplementation. However, whether probiotic supplements can reach the baby through the mother and reduce the incidence of neonatal jaundice has not been studied yet. Therefore, we aim to evaluate the effect of prenatal maternal probiotic supplementation on the incidence of neonatal jaundice.
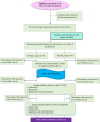
Methods and analysis: This is a randomised double-blind placebo-controlled clinical trial among 94 pregnant women (47 in each group) in a tertiary hospital in Hong Kong. Voluntary eligible participants will be recruited between 28 and 35 weeks of gestation. Computer-generated randomisation and allocation to either the intervention or control group will be carried out. Participants will take either one sachet of Vivomixx (450 billion colony-forming units per sachet) or a placebo per day until 1 week post partum. Neither the study participants nor researchers will know the randomisation and allocation. The intervention will be initiated at 36 weeks of gestation. Neonatal bilirubin level will be measured to determine the primary outcome (hyperbilirubinaemia) while the metagenomic microbiome profile of breast milk and maternal and infant stool samples as well as pregnancy outcomes will be secondary outcomes. Binary logistic and linear regressions will be carried out to assess the association of the microbiome data with different clinical outcomes.
Ethics and dissemination: Ethics approval is obtained from the Joint CUHK-NTEC Clinical Research Ethics Committee, Hong Kong (CREC Ref: 2023.100-T). Findings will be published in peer-reviewed journals and presented at international conferences.
Trial registration number: NCT06087874.
Keywords: Clinical Trial; Fetal medicine; Maternal medicine; NEONATOLOGY; OBSTETRICS; Prenatal diagnosis.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
The Probiotics in Pregnancy Study (PiP Study): rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy.BMC Pregnancy Childbirth. 2016 Jun 3;16(1):133. doi: 10.1186/s12884-016-0923-y. BMC Pregnancy Childbirth. 2016. PMID: 27255079 Free PMC article. Clinical Trial.
-
Effect of probiotic administration to breastfeeding mothers with very low birthweight neonates on some neonatal and maternal outcomes: study protocol for a randomised, double-blind, placebo-controlled trial.BMJ Open. 2024 Aug 29;14(8):e079526. doi: 10.1136/bmjopen-2023-079526. BMJ Open. 2024. PMID: 39209790 Free PMC article.
-
Evaluation of efficacy of oral calcium phosphate as an adjunct to standard-of-care regular phototherapy in cases of neonatal jaundice: a hospital-based double-blind, randomised, placebo-controlled trial.BMJ Paediatr Open. 2024 Oct 11;8(1):e002902. doi: 10.1136/bmjpo-2024-002902. BMJ Paediatr Open. 2024. PMID: 39395818 Free PMC article. Clinical Trial.
-
Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants.Cochrane Database Syst Rev. 2018 Dec 12;12(12):CD012519. doi: 10.1002/14651858.CD012519.pub2. Cochrane Database Syst Rev. 2018. PMID: 30548483 Free PMC article.
-
Multiple-micronutrient supplementation for women during pregnancy.Cochrane Database Syst Rev. 2019 Mar 14;3(3):CD004905. doi: 10.1002/14651858.CD004905.pub6. Cochrane Database Syst Rev. 2019. PMID: 30873598 Free PMC article.
Cited by
-
Maternal glycemic profiles during pregnancy and predelivery correlate with neonatal glucose homeostasis and jaundice risk: a prospective cohort study.Transl Pediatr. 2024 Nov 30;13(11):2012-2025. doi: 10.21037/tp-24-356. Epub 2024 Nov 26. Transl Pediatr. 2024. PMID: 39649657 Free PMC article.
-
Gut microbiota dysbiosis in infantile cholestatic hepatopathy.Front Pediatr. 2025 Mar 24;13:1547958. doi: 10.3389/fped.2025.1547958. eCollection 2025. Front Pediatr. 2025. PMID: 40196165 Free PMC article.
References
-
- Gartner LM. Neonatal jaundice. Pediatr Rev 1994;15:422–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
