Design and synthesis of a library of C8-substituted sulfamidoadenosines to probe bacterial permeability
- PMID: 38851357
- PMCID: PMC11361631
- DOI: 10.1016/j.bmcl.2024.129844
Design and synthesis of a library of C8-substituted sulfamidoadenosines to probe bacterial permeability
Abstract
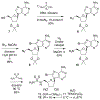
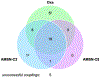
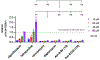
Gram-negative bacteria pose a major challenge in antibiotic drug discovery because their cell envelope presents a permeability barrier that affords high intrinsic resistance to small-molecule drugs. The identification of correlations between chemical structure and Gram-negative permeability would thus enable development of predictive tools to facilitate antibiotic discovery. Toward this end, have advanced a library design paradigm in which various chemical scaffolds are functionalized at different regioisomeric positions using a uniform reagent set. This design enables decoupling of scaffold, regiochemistry, and substituent effects upon Gram-negative permeability of these molecules. Building upon our recent synthesis of a library of C2-substituted sulfamidoadenosines, we have now developed an efficient synthetic route to an analogous library of regioisomeric C8-substituted congeners. The C8 library samples a region of antibiotic-relevant chemical space that is similar to that addressed by the C2 library, but distinct from that sampled by a library of analogously substituted oxazolidinones. Selected molecules were tested for accumulation in Escherichia coli in a pilot analysis, setting the stage for full comparative evaluation of these libraries in the future.
Keywords: Antibiotic resistance; Diversity-oriented synthesis; Drug discovery; Gram-negative bacteria; Nucleoside analogue.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: B.S.S. is a former employee of Merck Sharpe & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. D.S.T. has received in-kind research support from Merck Sharpe & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA as a collaborating institution on this project and, in the last 3 years, has held equity interests in Merck & Co., Inc., Rahway, NJ, USA.
Figures


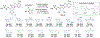

References
-
- Brown ED, Wright GD. Nature 2016;529:336. - PubMed
-
- Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, Han C, Bisignano C, Rao P, Wool E. Lancet 2022;399:629.
-
- ”Antibiotic Resistance Threats in the United States, 2019,” Centers for Disease Control and Prevention, 2019; https://www.cdc.gov/drugresistance/biggest-threats.html.
-
- ”COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022,” Centers for Disease Control and Prevention, 2022; https://www.cdc.gov/drugresistance/covid19.html.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

