Accuracy of cobas MTB and MTB-RIF/INH for Detection of Mycobacterium tuberculosis and Drug Resistance
- PMID: 38851386
- PMCID: PMC11298579
- DOI: 10.1016/j.jmoldx.2024.05.004
Accuracy of cobas MTB and MTB-RIF/INH for Detection of Mycobacterium tuberculosis and Drug Resistance
Abstract
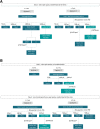
This study evaluated the performance of cobas MTB and cobas MTB-RIF/INH for the diagnosis of tuberculosis and detection of rifampicin (RIF) and isoniazid (INH) resistance. Adults presenting with pulmonary tuberculosis symptoms were recruited in South Africa, Moldova, and India. Performance of cobas MTB was assessed against culture, whereas cobas MTB-RIF/INH was assessed using phenotypic drug susceptibility testing and whole-genome sequencing as composite reference standards. Xpert MTB/RIF (Xpert) or Xpert MTB/RIF Ultra (Ultra) was used as a comparator. The overall sensitivity and specificity of cobas MTB were 95% (95% CI, 93%-96%) and 96% (95% CI, 95%-97%). Among smear-negatives, the sensitivity of cobas MTB was 75% (95% CI, 66%-83%). Among participants tested with both cobas MTB and Xpert, sensitivity was 96% (95% CI, 94%-97%) for cobas MTB and 95% (95% CI, 93%-97%) for Xpert. Among participants tested with both cobas MTB and Ultra, sensitivity was 88% (95% CI, 81%-92%) for cobas MTB and 89% (95% CI, 83%-93%) for Ultra. Sensitivity and specificity of cobas MTB-RIF/INH for RIF and INH detection were 90% (95% CI, 84%-94%) and 100% (95% CI, 99%-100%), and 89% (95% CI, 84%-93%) and 99.5% (95% CI, 98%-100%), respectively. The cobas MTB and cobas MTB-RIF/INH assays exhibited high performance in a diverse population and present a suitable option for molecular detection of tuberculosis and RIF and INH resistance.
Copyright © 2024 Association for Molecular Pathology and American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure Statement M.d.V., A.P.-N., A.M., S.O., P.N., and M.R. are employees of FIND. S.G.S., J.M., I.K., and A.P. were employed by FIND at the time of the study. S.S., S.S.C., and K.D. are employees of FIND, India. F.P.M. changed employment from Research Center Borstel (Borstel, Germany) to Roche (Rotkreuz, Switzerland) after the completion of the study in July 2022. (A.M., S.O., P.N., S.S., S.S.C., J.M., I.K., and A.P. are Study Group members.)
Figures







References
-
- World Health Organization; Geneva, Switzerland: 2023. WHO: Global Tuberculosis Report 2023.
-
- Georghiou S.B., Schumacher S.G., Rodwell T.C., Colman R.E., Miotto P., Gilpin C., Ismail N., Rodrigues C., Warren R., Weyer K., Zignol M., Arafah S., Cirillo D.M., Denkinger C.M. Guidance for studies evaluating the accuracy of rapid tuberculosis drug-susceptibility tests. J Infect Dis. 2019;220:S126–S135. - PubMed
-
- World Health Organization; Geneva, Switzerland: 2021. WHO: WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis: Rapid Diagnostics for Tuberculosis Detection, 2021 Update.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

