Spatial Intracranial Pressure Fields Driven by Blast Overpressure in Rats
- PMID: 38851659
- PMCID: PMC11402848
- DOI: 10.1007/s10439-024-03544-7
Spatial Intracranial Pressure Fields Driven by Blast Overpressure in Rats
Abstract
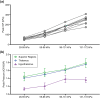
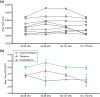
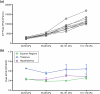
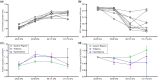
Free-field blast exposure imparts a complex, dynamic response within brain tissue that can trigger a cascade of lasting neurological deficits. Full body mechanical and physiological factors are known to influence the body's adaptation to this seemingly instantaneous insult, making it difficult to accurately pinpoint the brain injury mechanisms. This study examined the intracranial pressure (ICP) profile characteristics in a rat model as a function of blast overpressure magnitude and brain location. Metrics such as peak rate of change of pressure, peak pressure, rise time, and ICP frequency response were found to vary spatially throughout the brain, independent of blast magnitude, emphasizing unique spatial pressure fields as a primary biomechanical component to blast injury. This work discusses the ICP characteristics and considerations for finite element models, in vitro models, and translational in vivo models to improve understanding of biomechanics during primary blast exposure.
Keywords: Biomechanics; Blast-induced traumatic brain injury; In vivo; Injury characterization; Intracranial pressure.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors disclose no conflicts of interest.
Figures



Similar articles
-
Rat injury model under controlled field-relevant primary blast conditions: acute response to a wide range of peak overpressures.J Neurotrauma. 2013 Jul 1;30(13):1147-60. doi: 10.1089/neu.2012.2652. Epub 2013 Jun 28. J Neurotrauma. 2013. PMID: 23362798 Free PMC article.
-
A 3-D Rat Brain Model for Blast-Wave Exposure: Effects of Brain Vasculature and Material Properties.Ann Biomed Eng. 2019 Sep;47(9):2033-2044. doi: 10.1007/s10439-019-02277-2. Epub 2019 May 3. Ann Biomed Eng. 2019. PMID: 31054004 Free PMC article.
-
Effects of Exposure to Blast Overpressure on Intracranial Pressure and Blood-Brain Barrier Permeability in a Rat Model.PLoS One. 2016 Dec 1;11(12):e0167510. doi: 10.1371/journal.pone.0167510. eCollection 2016. PLoS One. 2016. PMID: 27907158 Free PMC article.
-
In-vitro approaches for studying blast-induced traumatic brain injury.J Neurotrauma. 2009 Jun;26(6):861-76. doi: 10.1089/neu.2008.0645. J Neurotrauma. 2009. PMID: 19397424 Free PMC article. Review.
-
Blast-induced brain injury and posttraumatic hypotension and hypoxemia.J Neurotrauma. 2009 Jun;26(6):877-87. doi: 10.1089/neu.2007.0439. J Neurotrauma. 2009. PMID: 18447627 Review.
Cited by
-
High-rate mechano-stimulation alters proliferation- and maturation-related signaling of oligodendrocyte precursor cells in a 3D hydrogel.Mechanobiol Med. 2025 Mar 11;3(3):100126. doi: 10.1016/j.mbm.2025.100126. eCollection 2025 Sep. Mechanobiol Med. 2025. PMID: 40519865 Free PMC article.
-
Modeling biomarker kinetics of Aβ levels in serum following blast.Front Neurol. 2025 Apr 4;16:1548589. doi: 10.3389/fneur.2025.1548589. eCollection 2025. Front Neurol. 2025. PMID: 40255887 Free PMC article.
References
-
- McNeil, E., Z. Bailey, A. Guettler and P. VandeVord. Blast traumatic brain injury mechanisms. In: Neurotrauma: a comprehensive textbook on traumatic brain injury and spinal cord injury, 2018, p. 111
-
- Du, Z., Z. Li, P. Wang, X. Wang, J. Zhang, Z. Zhuang, and Z. Liu. Revealing the effect of skull deformation on intracranial pressure variation during the direct interaction between blast wave and surrogate head. Ann. Biomed. Eng. 50:1038–1052, 2022. 10.1007/s10439-022-02982-5. 10.1007/s10439-022-02982-5 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

