The efficacy and safety of PARP inhibitors in mCRPC with HRR mutation in second-line treatment: a systematic review and bayesian network meta-analysis
- PMID: 38851712
- PMCID: PMC11162002
- DOI: 10.1186/s12885-024-12388-2
The efficacy and safety of PARP inhibitors in mCRPC with HRR mutation in second-line treatment: a systematic review and bayesian network meta-analysis
Abstract
Background: Poly (ADP- ribose) polymerase inhibitors (PARPi) has been increasingly adopted for metastatic castration-resistance prostate cancer (mCRPC) patients with homologous recombination repair deficiency (HRD). However, it is unclear which PARPi is optimal in mCRPC patients with HRD in 2nd -line setting.
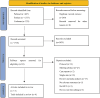
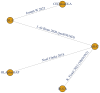
Method: We conducted a systematic review of trials regarding PARPi- based therapies on mCRPC in 2nd -line setting and performed a Bayesian network meta-analysis (NMA). Radiographic progression-free survival (rPFS) was assessed as primary outcome. PSA response and adverse events (AEs) were evaluated as secondary outcomes. Subgroup analyses were performed according to specific genetic mutation.
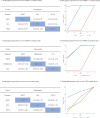
Results: Four RCTs comprised of 1024 patients (763 harbored homologous recombination repair (HRR) mutations) were identified for quantitative analysis. Regarding rPFS, olaparib monotherapy, rucaparib and cediranib plus olaparib showed significant improvement compared with ARAT. Olaparib plus cediranib had the highest surface under cumulative ranking curve (SUCRA) scores (87.5%) for rPFS, followed by rucaparib, olaparib and olaparib plus abiraterone acetate prednisone. For patients with BRCA 1/2 mutations, olaparib associated with the highest probability (98.1%) of improved rPFS. For patients with BRCA-2 mutations, olaparib and olaparib plus cediranib had similar efficacy. However, neither olaparib nor rucaparib showed significant superior effectiveness to androgen receptor-axis-targeted therapy (ARAT) in patients with ATM mutations. For safety, olaparib showed significantly lower ≥ 3 AE rate compared with cediranib plus olaparib (RR: 0.72, 95% CI: 0.51, 0.97), while olaparib plus cediranib was associated with the highest risk of all-grade AE.
Conclusion: PARPi-based therapy showed considerable efficacy for mCRPC patients with HRD in 2nd -line setting. However, patients should be treated accordingly based on their genetic background as well as the efficacy and safety of the selected regimen.
Trial registration: CRD42023454079.
Keywords: Adverse events; Homologous recombination deficiency; Metastatic castration-resistance prostate cancer; Poly (ADP- ribose) polymerase inhibitors; Progression-free survival.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ryan CJ, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–60. doi: 10.1016/S1470-2045(14)71205-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NSFC 82203110, 82172785, and 81974398/National Natural Science Foundation of China
- 2021YFS0119/Sichuan Province Science and Technology Support Program
- ZYJC21020, ZYGD22004/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- 2022-I2M-C&T-B-098/Clinical and Translational Medicine Research Project, Chinese Academy of Medical Sciences
- X-J-2020-016/Bethune Foundation, Oncology Basic Research Program
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

