Predictors of short-term anxiety outcome in subthalamic stimulation for Parkinson's disease
- PMID: 38851717
- PMCID: PMC11162430
- DOI: 10.1038/s41531-024-00701-6
Predictors of short-term anxiety outcome in subthalamic stimulation for Parkinson's disease
Abstract
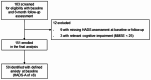
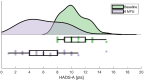
The effects of subthalamic nucleus deep brain stimulation (STN-DBS) on anxiety in Parkinson's disease (PD) are understudied. We identified clinical predictors of STN-DBS effects on anxiety in this study. In this prospective, open-label, multicentre study, we assessed patients with anxiety undergoing STN-DBS for PD preoperatively and at 6-month follow-up postoperatively. We assessed the Hospital Anxiety and Depression Scale (HADS-anxiety and depression subscales), Unified PD Rating Scale-motor examination, Scales for Outcomes in PD-motor (SCOPA-M)-activities of daily living (ADL) and -motor complications, Non-Motor Symptom Scale (NMSS), PDQuestionnaire-8 (PDQ-8), and levodopa-equivalent daily dose. We tested changes at follow-up with Wilcoxon signed-rank test and corrected for multiple comparisons (Bonferroni method). We identified patients with a clinically relevant anxiety improvement of anxiety based on a designated threshold of ½ standard deviation of baseline HADS-anxiety. Moreover, we investigated predictors of HADS-anxiety changes with correlations and linear regressions. We included 50 patients with clinically relevant baseline anxiety (i.e., HADS-anxiety ≥ 8) aged 63.1 years ± 8.3 with 10.4 years ± 4.5 PD duration. HADS-anxiety improved significantly at 6-month follow-up as 80% of our cohort experienced clinically relevant anxiety improvement. In predictor analyses, worse baseline SCOPA-ADL and NMSS-urinary domain were associated with greater HADS-anxiety improvements. HADS-anxiety and PDQ-8 changes correlated moderately. Worse preoperative ADL and urinary symptoms predicted favourable postoperative anxiety outcome, which in turn was directly proportionate to greater QoL improvement. This study highlights the importance of detailed anxiety assessments alongside other non-motor and motor symptoms when advising and monitoring patients undergoing STN-DBS for PD.
© 2024. The Author(s).
Conflict of interest statement
Anna Sauerbier is funded by the Gusyk program and the Advanced Cologne Clinician Scientist program of the Medical Faculty of the University of Cologne and has received funding from the Prof. Klaus Thiemann Foundation. Johanna Herberg reports no financial disclosures. Vasilija Stopic is funded as part of the Advanced Cologne Clinician Scientist program. Philipp A. Loehrer was funded by the SUCCESS-Program of the University Hospital Marburg and the Parkinson’s Foundation. He reports travel grants from AbbVie. Keyoumars Ashkan has received honoraria for educational meetings, travel and consultancy from Medtronic, St Jude Medical and Boston Scientific. Alexandra Rizos has received honorarium from UCB and was supported by a grant from Medtronic. Stefanie T. Jost has received funding from the Prof. Klaus Thiemann Foundation. Jan Niklas Petry-Schmelzer has been funded by the Cologne Clinician Scientist Program (CCSP)/ Faculty of Medicine/ University of Cologne. Funded by the German Research Foundation (DFG, FI 773/15-1) and received travel grants from Boston Scientific. Alexandra Gronostay reports no financial disclosures. Veerle Visser-Vandewalle is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 431549029 – SFB 1451, is a member of the advisory boards and reports consultancies for Medtronic, Boston Scientific, Abbott, Insightec and LivaNova. Julian Evans reports no financial disclosures. Christopher Nimsky is consultant for Brainlab and received speaker’s honoraria. Gereon R. Fink is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 431549029 – SFB 1451. GRF serves as an editorial board member of Cortex, Neurological Research and Practice, NeuroImage: Clinical, Zeitschrift für Neuropsychologie, and DGNeurologie; receives royalties from the publication of the books Funktionelle MRT in Psychiatrie und Neurologie, Neurologische Differentialdiagnose, and SOP Neurologie; receives royalties from the publication of the neuropsychological tests KAS and Köpps; received honoraria for speaking engagements from Bayer, Desitin, DGN, Ergo DKV, Forum für medizinische Fortbildung FomF GmbH, GSK, Medica Academy Messe Düsseldorf, Medicbrain Healthcare, Novartis, Pfizer, and Sportärztebund NRW. Angelo Antonini reports personal consultancy fees from Zambon, AbbVie, Boehringer Ingelheim, GE, Neuroderm, Biogen, Bial, EVER Neuro Pharma, Therevance, Vectura grants from Chiesi Pharmaceuticals, Lundbeck, Horizon 2020 - PD_Pal Grant 825785, Ministry of Education University and Research (MIUR) Grant ARS01_01081, owns Patent WO2015110261-A1, owns shares from PD Neurotechnology Limited. Pablo Martinez-Martin has received honoraria from Editorial Viguera and Movement Disorder Society for lecturing in courses; from AbbVie for speaking in experts’ meetings and from AbbVie and Zambon for participating in the Advisory Board of epidemiological studies. License fee payments for the King’s Parkinson’s Disease Pain Scale, and grants from the International Parkinson and Movement Disorder Society for development and validation of the MDS-Non-Motor Symptoms Scale. Monty Silverdale has received honoraria from Bial, Britannia and Medtronic. Daniel Weintraub reports no financial disclosures. Anette Schrag reports no financial disclosures. K. Ray Chaudhuri has received funding from Parkinson’s UK, NIHR, UCB, and the European Union; he received honoraria from UCB, Abbott, Britannia, US Worldmeds, and Otsuka Pharmaceuticals; and acted as a consultant for AbbVie, UCB, and Britannia. Lars Timmermann reports grants, personal fees and non-financial support from SAPIENS Steering Brain Stimulation, Medtronic, Boston Scientific and St. Jude Medical. Haidar S. Dafsari reports funding of his work by the EU Joint Programme—Neurodegenerative Disease Research (JPND), the Prof. Klaus Thiemann Foundation, the Felgenhauer Foundation, and the Koeln Fortune Program, and honoraria by Boston Scientific, Medtronic, Bial, and Stadapharm.
Figures
References
-
- Barone, P. et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord.24, 10.1002/mds.22643 (2009). - PubMed
LinkOut - more resources
Full Text Sources

