The Hospital Burden of Flu in Italy: a retrospective study on administrative data from season 2014-2015 to 2018-2019
- PMID: 38851739
- PMCID: PMC11162570
- DOI: 10.1186/s12879-024-09446-2
The Hospital Burden of Flu in Italy: a retrospective study on administrative data from season 2014-2015 to 2018-2019
Abstract
Background: Every year in Italy, influenza affects about 4 million people. Almost 5% of them are hospitalised. During peak illness, enormous pressure is placed on healthcare and economic systems. This study aims to quantify the clinical and economic burden of severe influenza during 5 epidemic seasons (2014-2019) from administrative claims data.
Methods: Patients hospitalized with a diagnosis of influenza between October 2014, and April 2019, were analyzed. Clinical characteristics and administrative information were retrieved from health-related Administrative Databases (ADs) of 4 Italian Local Health Units (LHUs). The date of first admission was set as the Index Date (ID). A follow-up period of six months after ID was considered to account for complications and re-hospitalizations, while a lookback period (2 years before ID) was set to assess the prevalence of underlying comorbidities.
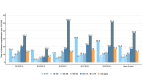
Results: Out of 2,333 patients with severe influenza, 44.1% were adults ≥ 65, and 25.6% young individuals aged 0-17. 46.8% had comorbidities (i.e., were at risk), mainly cardiovascular and metabolic diseases (45.3%), and chronic conditions (24.7%). The highest hospitalization rates were among the elderly (≥ 75) and the young individuals (0-17), and were 37.6 and 19.5/100,000 inhabitants/year, respectively. The average hospital stay was 8 days (IQR: 14 - 4). It was higher for older individuals (≥ 65 years, 11 days, [17 - 6]) and for those with comorbidities (9 days, [16 - 6]), p-value < 0.001. Similarly, mortality was higher in elderly and those at risk (p-value < 0.001). Respiratory complications occurred in 12.7% of patients, and cardiovascular disorders in 5.9%. Total influenza-related costs were €9.7 million with hospitalization accounting for 95% of them. 47.3% of hospitalization costs were associated with individuals ≥ 65 and 52.9% with patients at risk. The average hospitalisation cost per patient was € 4,007.
Conclusions: This retrospective study showed that during the 2014-2019 influenza seasons in Italy, individuals of extreme ages and those with pre-existing medical conditions, were more likely to be hospitalized with severe influenza. Together with complications and ageing, they worsen patient's outcome and may lead to a prolonged hospitalization, thus increasing healthcare utilization and costs. Our data generate real-world evidence on the burden of influenza, useful to inform public health decision-making.
Keywords: Administrative database; Burden; Comorbidities; Complications; Costs; Hospitalization; Influenza; Local health units; Secondary health care.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: E. X. have disclosed that in the last two years she took part in advisory boards organized by: Eli Lilly, Regeneron Pharmaceuticals, Diurnal Limited, GSK Spa, Incyte Biosciences Italy Srl, Bristol-Mayers Squibb & Affiliates, Blueprint Medicines Italy Srl, Bayer AG, Takeda Pharmaceutical International AG, Astellas Pharma Europe Ltd, Takeda Italia Spa, Actelion Pharmaceuticals Ltd, Genzyme Europe BV, Gilead Sciences Srl, Sanofi Spa and other Italian and international consulting companies. She disclosed that she was engaged in consulting activities with Gilead Sciences Srl, Zambon Italia, Insmed Italy Srl. For the activities mentioned above, all unrelated to this article, she disclosed to have received fees. M.R. G. have disclosed that she received a fee by Sanofi, for a lecture.R. C., S. F., and E. L. have disclosed that they are employees of IQVIA Solutions SRL. M.V.A. and L. Z. are employees of Sanofi, the manufacturer of influenza vaccines, and may hold shares and/or stock options in the company.The remaining authors declare that they have no competing interests. E. X. have disclosed that in the last two years she took part in advisory boards organized by: Eli Lilly, Regeneron Pharmaceuticals, Diurnal Limited, GSK Spa, Incyte Biosciences Italy Srl, Bristol-Mayers Squibb & Affiliates, Blueprint Medicines Italy Srl, Bayer AG, Takeda Pharmaceutical International AG, Astellas Pharma Europe Ltd, Takeda Italia Spa, Actelion Pharmaceuticals Ltd, Genzyme Europe BV, Gilead Sciences Srl, Sanofi Spa and other Italian and international consulting companies. She disclosed that she was engaged in consulting activities with Gilead Sciences Srl, Zambon Italia, Insmed Italy Srl. For the activities mentioned above, all unrelated to this article, she disclosed to have received fees. M.R. G. have disclosed that she received a fee by Sanofi, for a lecture. R. C., S. F., and E. L. have disclosed that they are employees of IQVIA Solutions SRL. M.V.A. and L. Z. are employees of Sanofi, the manufacturer of influenza vaccines, and may hold shares and/or stock options in the company. The remaining authors declare that they have no competing interests.
Figures


References
-
- CDC, Centers for Disease Control and Prevention Types of Influenza Viruses. About Flu. https://www.cdc.gov/flu/about/viruses/types.htm#:~:text=There%20are%20fo.... Accessed 20 November 2023.
-
- EpiCentro. Influenza epidemiologia in Italia. https://www.epicentro.iss.it/influenza/epidemiologia-italia. Accessed 20 November 2023.
-
- Boccalini S, Fiaschi P, Panatto D, Amicizia D, Lai PL, Gasparini R, Bechini A, Bonanni P. Epidemiologia dell’influenza stagionale negli anziani: Un’analisi dei dati italiani sulla sorveglianza clinico-epidemiologica e virologica. Ital J Public Health. 2017;6:4.
-
- Influenza. (Seasonal). https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). Accessed 20 November 2023.
-
- CDC, Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases, Chap. 12: Influenza. https://www.cdc.gov/vaccines/pubs/pinkbook/flu.html#impact. Accessed 11 May 2023.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

