Carbon-wise utilization of lignin-related compounds by synergistically employing anaerobic and aerobic bacteria
- PMID: 38851749
- PMCID: PMC11161944
- DOI: 10.1186/s13068-024-02526-0
Carbon-wise utilization of lignin-related compounds by synergistically employing anaerobic and aerobic bacteria
Abstract
Background: Lignin is a highly abundant but strongly underutilized natural resource that could serve as a sustainable feedstock for producing chemicals by microbial cell factories. Because of the heterogeneous nature of the lignin feedstocks, the biological upgrading of lignin relying on the metabolic routes of aerobic bacteria is currently considered as the most promising approach. However, the limited substrate range and the inefficient catabolism of the production hosts hinder the upgrading of lignin-related aromatics. Particularly, the aerobic O-demethylation of the methoxyl groups in aromatic substrates is energy-limited, inhibits growth, and results in carbon loss in the form of CO2.
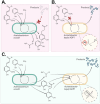
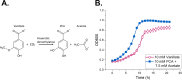
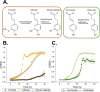
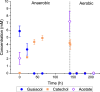
Results: In this study, we present a novel approach for carbon-wise utilization of lignin-related aromatics by the integration of anaerobic and aerobic metabolisms. In practice, we employed an acetogenic bacterium Acetobacterium woodii for anaerobic O-demethylation of aromatic compounds, which distinctively differs from the aerobic O-demethylation; in the process, the carbon from the methoxyl groups is fixed together with CO2 to form acetate, while the aromatic ring remains unchanged. These accessible end-metabolites were then utilized by an aerobic bacterium Acinetobacter baylyi ADP1. By utilizing this cocultivation approach, we demonstrated an upgrading of guaiacol, an abundant but inaccessible substrate to most microbes, into a plastic precursor muconate, with a nearly equimolar yields (0.9 mol/mol in a small-scale cultivation and 1.0 mol/mol in a one-pot bioreactor cultivation). The process required only a minor genetic engineering, namely a single gene knock-out. Noticeably, by employing a metabolic integration of the two bacteria, it was possible to produce biomass and muconate by utilizing only CO2 and guaiacol as carbon sources.
Conclusions: By the novel approach, we were able to overcome the issues related to aerobic O-demethylation of methoxylated aromatic substrates and demonstrated carbon-wise conversion of lignin-related aromatics to products with yields unattainable by aerobic processes. This study highlights the power of synergistic integration of distinctive metabolic features of bacteria, thus unlocking new opportunities for harnessing microbial cocultures in upgrading challenging feedstocks.
Keywords: Acetobacterium woodii; Acinetobacter baylyi ADP1; Cis,cis-muconate; O-demethylation; Biological lignin upgrading; Guaiacol; Metabolic integration; Synergistic cocultures.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Catalytic Conversion of Lignin into Valuable Chemicals: Full Utilization of Aromatic Nuclei and Side Chains.Acc Chem Res. 2023 Dec 19;56(24):3558-3571. doi: 10.1021/acs.accounts.3c00514. Epub 2023 Nov 29. Acc Chem Res. 2023. PMID: 38029298
-
Engineering Acinetobacter baylyi ADP1 for mevalonate production from lignin-derived aromatic compounds.Metab Eng Commun. 2021 May 24;13:e00173. doi: 10.1016/j.mec.2021.e00173. eCollection 2021 Dec. Metab Eng Commun. 2021. PMID: 34430203 Free PMC article.
-
Characterization of Highly Ferulate-Tolerant Acinetobacter baylyi ADP1 Isolates by a Rapid Reverse Engineering Method.Appl Environ Microbiol. 2022 Jan 25;88(2):e0178021. doi: 10.1128/AEM.01780-21. Epub 2021 Nov 17. Appl Environ Microbiol. 2022. PMID: 34788063 Free PMC article.
-
Opportunities and challenges in biological lignin valorization.Curr Opin Biotechnol. 2016 Dec;42:40-53. doi: 10.1016/j.copbio.2016.02.030. Epub 2016 Mar 11. Curr Opin Biotechnol. 2016. PMID: 26974563 Review.
-
Unleashing the capacity of Rhodococcus for converting lignin into lipids.Biotechnol Adv. 2024 Jan-Feb;70:108274. doi: 10.1016/j.biotechadv.2023.108274. Epub 2023 Oct 31. Biotechnol Adv. 2024. PMID: 37913947 Review.
Cited by
-
Analysis of detoxification kinetics and end products of furan aldehydes in Acinetobacter baylyi ADP1.Sci Rep. 2024 Nov 29;14(1):29678. doi: 10.1038/s41598-024-81124-4. Sci Rep. 2024. PMID: 39613800 Free PMC article.
References
-
- Guadix-Montero S, Sankar M. Review on catalytic cleavage of C-C inter-unit linkages in lignin model compounds: towards lignin depolymerisation. Top Catal. 2018;61(3):183–198.
-
- Laurichesse S, Avérous L. Chemical modification of lignins: towards biobased polymers. Prog Polym Sci. 2014;39(7):1266–1290.
-
- Chio C, Sain M, Qin W. Lignin utilization: a review of lignin depolymerization from various aspects. Renew Sustain Energy Rev. 2019;1(107):232–249.
-
- Ralph J, Lapierre C, Boerjan W. Lignin structure and its engineering. Curr Opin Biotechnol. 2019;1(56):240–249. - PubMed
-
- Wells T, Ragauskas AJ. Biotechnological opportunities with the β-ketoadipate pathway. Trends Biotechnol. 2012;30(12):627–637. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
