Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury
- PMID: 38852200
- PMCID: PMC11219935
- DOI: 10.1016/j.redox.2024.103194
Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury
Erratum in
-
Corrigendum to "Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury" [Redox Biol. 74 (2024) 103194].Redox Biol. 2025 May;82:103616. doi: 10.1016/j.redox.2025.103616. Epub 2025 Apr 10. Redox Biol. 2025. PMID: 40216567 Free PMC article. No abstract available.
Abstract
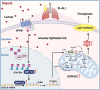
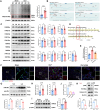
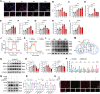
Elevated lactate levels are a significant biomarker of sepsis and are positively associated with sepsis-related mortality. Sepsis-associated lung injury (ALI) is a leading cause of poor prognosis in clinical patients. However, the underlying mechanisms of lactate's involvement in sepsis-associated ALI remain unclear. In this study, we demonstrate that lactate regulates N6-methyladenosine (m6A) modification levels by facilitating p300-mediated H3K18la binding to the METTL3 promoter site. The METTL3-mediated m6A modification is enriched in ACSL4, and its mRNA stability is regulated through a YTHDC1-dependent pathway. Furthermore, short-term lactate stimulation upregulates ACSL4, which promotes mitochondria-associated ferroptosis. Inhibition of METTL3 through knockdown or targeted inhibition effectively suppresses septic hyper-lactate-induced ferroptosis in alveolar epithelial cells and mitigates lung injury in septic mice. Our findings suggest that lactate induces ferroptosis via the GPR81/H3K18la/METTL3/ACSL4 axis in alveolar epithelial cells during sepsis-associated ALI. These results reveal a histone lactylation-driven mechanism inducing ferroptosis through METTL3-mediated m6A modification. Targeting METTL3 represents a promising therapeutic strategy for patients with sepsis-associated ALI.
Keywords: Ferroptosis; Histone lactylation; N6- methyladenosine; Sepsis-associated acute lung injury.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
EP300-mediated H3K18la regulation of METTL3 promotes macrophage ferroptosis and atherosclerosis through the m6A modification of SLC7A11.Biochim Biophys Acta Gen Subj. 2025 Aug;1869(9):130838. doi: 10.1016/j.bbagen.2025.130838. Epub 2025 Jun 28. Biochim Biophys Acta Gen Subj. 2025. PMID: 40588140
-
METTL3-mediated m6A Modification Promotes miR-221-3p Expression to Exacerbate Ischemia/Reperfusion-Induced Acute Lung Injury.J Biochem Mol Toxicol. 2025 Apr;39(4):e70235. doi: 10.1002/jbt.70235. J Biochem Mol Toxicol. 2025. PMID: 40127211
-
METTL3-m6A methylation inhibits the proliferation and viability of type II alveolar epithelial cells in acute lung injury by enhancing the stability and translation efficiency of Pten mRNA.Respir Res. 2024 Jul 15;25(1):276. doi: 10.1186/s12931-024-02894-z. Respir Res. 2024. PMID: 39010105 Free PMC article.
-
METTL3-mediated m6A modification in sepsis: current evidence and future perspectives.Epigenomics. 2025 Jun;17(9):611-623. doi: 10.1080/17501911.2025.2494983. Epub 2025 Apr 19. Epigenomics. 2025. PMID: 40251974 Review.
-
RNA m6A methylation regulators in sepsis.Mol Cell Biochem. 2024 Sep;479(9):2165-2180. doi: 10.1007/s11010-023-04841-w. Epub 2023 Sep 2. Mol Cell Biochem. 2024. PMID: 37659034 Review.
Cited by
-
Lactylation: From Molecular Insights to Disease Relevance.Biomolecules. 2025 Jun 3;15(6):810. doi: 10.3390/biom15060810. Biomolecules. 2025. PMID: 40563450 Free PMC article. Review.
-
Review of research progress in sepsis-associated acute kidney injury.Front Mol Biosci. 2025 Jul 11;12:1603392. doi: 10.3389/fmolb.2025.1603392. eCollection 2025. Front Mol Biosci. 2025. PMID: 40718792 Free PMC article. Review.
-
Utilizing integrated bioinformatics and machine learning approaches to elucidate biomarkers linking sepsis to purine metabolism-associated genes.Sci Rep. 2025 Jan 2;15(1):353. doi: 10.1038/s41598-024-82998-0. Sci Rep. 2025. PMID: 39747316 Free PMC article.
-
MiR-125b-5p ameliorates ventilator-induced lung injury in rats by suppressing ferroptosis via the regulation of the Keap1/Nrf2/GPX4 signaling pathway.Sci Rep. 2025 Jul 1;15(1):21199. doi: 10.1038/s41598-025-04730-w. Sci Rep. 2025. PMID: 40595901 Free PMC article.
-
The Emerging Role of m6A and Programmed Cell Death in Cardiovascular Diseases.Biomolecules. 2025 Feb 8;15(2):247. doi: 10.3390/biom15020247. Biomolecules. 2025. PMID: 40001550 Free PMC article. Review.
References
-
- Nolt B., Tu F., Wang X., et al. Lactate and immunosuppression in sepsis. Shock. 2018;49:120–125. doi: 10.1097/SHK.0000000000000958. https://www.ncbi.nlm.nih.gov/pubmed/28767543 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

