Clinical neurophysiology in the treatment of movement disorders: IFCN handbook chapter
- PMID: 38852434
- PMCID: PMC11418354
- DOI: 10.1016/j.clinph.2024.05.007
Clinical neurophysiology in the treatment of movement disorders: IFCN handbook chapter
Abstract
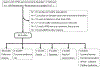
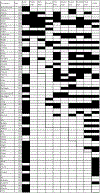
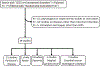
In this review, different aspects of the use of clinical neurophysiology techniques for the treatment of movement disorders are addressed. First of all, these techniques can be used to guide neuromodulation techniques or to perform therapeutic neuromodulation as such. Neuromodulation includes invasive techniques based on the surgical implantation of electrodes and a pulse generator, such as deep brain stimulation (DBS) or spinal cord stimulation (SCS) on the one hand, and non-invasive techniques aimed at modulating or even lesioning neural structures by transcranial application. Movement disorders are one of the main areas of indication for the various neuromodulation techniques. This review focuses on the following techniques: DBS, repetitive transcranial magnetic stimulation (rTMS), low-intensity transcranial electrical stimulation, including transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS), and focused ultrasound (FUS), including high-intensity magnetic resonance-guided FUS (MRgFUS), and pulsed mode low-intensity transcranial FUS stimulation (TUS). The main clinical conditions in which neuromodulation has proven its efficacy are Parkinson's disease, dystonia, and essential tremor, mainly using DBS or MRgFUS. There is also some evidence for Tourette syndrome (DBS), Huntington's disease (DBS), cerebellar ataxia (tDCS), and axial signs (SCS) and depression (rTMS) in PD. The development of non-invasive transcranial neuromodulation techniques is limited by the short-term clinical impact of these techniques, especially rTMS, in the context of very chronic diseases. However, at-home use (tDCS) or current advances in the design of closed-loop stimulation (tACS) may open new perspectives for the application of these techniques in patients, favored by their easier use and lower rate of adverse effects compared to invasive or lesioning methods. Finally, this review summarizes the evidence for keeping the use of electromyography to optimize the identification of muscles to be treated with botulinum toxin injection, which is indicated and widely performed for the treatment of various movement disorders.
Keywords: Botulinum toxin; Deep brain stimulation; Dystonia; Electromyography essential tremor; Focused ultrasound; Parkinson’s disease; Repetitive transcranial magnetic stimulation; Transcranial alternating current stimulation; Transcranial direct current stimulation.
Copyright © 2024 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Figures

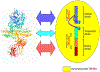





References
-
- Abo M, Shigematsu T, Hara H, Matsuda Y, Nimura A, Yamashita Y, et al. Efficacy and Safety of OnabotulinumtoxinA 400 Units in Patients with Post-Stroke Upper Limb Spasticity: Final Report of a Randomized, Double-Blind, Placebo-Controlled Trial with an Open-Label Extension Phase. Toxins (Basel) 2020;12(2):127. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

