EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
- PMID: 38852583
- PMCID: PMC11299976
- DOI: 10.1159/000539371
EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Erratum in
-
Erratum.Obes Facts. 2024;17(6):658. doi: 10.1159/000541386. Epub 2024 Sep 16. Obes Facts. 2024. PMID: 39284304 Free PMC article. No abstract available.
Abstract
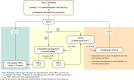
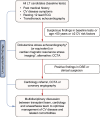
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously termed non-alcoholic fatty liver disease (NAFLD), is defined as steatotic liver disease (SLD) in the presence of one or more cardiometabolic risk factor(s) and the absence of harmful alcohol intake. The spectrum of MASLD includes steatosis, metabolic dysfunction-associated steatohepatitis (MASH, previously NASH), fibrosis, cirrhosis and MASH-related hepatocellular carcinoma (HCC). This joint EASL-EASD-EASO guideline provides an update on definitions, prevention, screening, diagnosis and treatment for MASLD. Case-finding strategies for MASLD with liver fibrosis, using non-invasive tests, should be applied in individuals with cardiometabolic risk factors, abnormal liver enzymes, and/or radiological signs of hepatic steatosis, particularly in the presence of type 2 diabetes (T2D) or obesity with additional metabolic risk factor(s). A stepwise approach using blood-based scores (such as FIB-4) and, sequentially, imaging techniques (such as transient elastography) is suitable to rule-out/in advanced fibrosis, which is predictive of liver-related outcomes. In adults with MASLD, lifestyle modification - including weight loss, dietary changes, physical exercise and discouraging alcohol consumption - as well as optimal management of comorbidities - including use of incretin-based therapies (e.g. semaglutide, tirzepatide) for T2D or obesity, if indicated - is advised. Bariatric surgery is also an option in individuals with MASLD and obesity. If locally approved and dependent on the label, adults with non-cirrhotic MASH and significant liver fibrosis (stage ≥2) should be considered for a MASH-targeted treatment with resmetirom, which demonstrated histological effectiveness on steatohepatitis and fibrosis with an acceptable safety and tolerability profile. No MASH-targeted pharmacotherapy can currently be recommended for the cirrhotic stage. Management of MASH-related cirrhosis includes adaptations of metabolic drugs, nutritional counselling, surveillance for portal hypertension and HCC, as well as liver transplantation in decompensated cirrhosis.
© 2024 The Author(s). Published by Elsevier BV on behalf of European Association for the Study of the Liver (EASL) and by S Karger AG.
Conflict of interest statement
The authors would like to thank the members of the Delphi Panel of this Clinical Practice Guideline for their valuable contribution:
Quentin Anstee, Marco Arrese, Heike Bantel, Giulia Besutti, Jérôme Boursier, Christopher Byrne, Ali Canbay, Cyrielle Caussy, Helena Cortez-Pinto, Mattias Ekstedt, Mirto Foletto, Jacob George, Liana Gheorghe, Isabel Graupera, Hannes Hagström, Kate Hallsworth, Onno Holleboom, Achim Kautz, Marko Korenjak, Karoline Lackner, Christos Lionis, Giulio Marchesini, Juris J. Meier, Juan M. Mendive, Luca Miele, Geltrude Mingrone, J. Bernadette Moore, Philip Newsome, George Papatheodoridis, Valerie Paradis, Gianluca Perseghin, Ralph Peterli, Salvatore Petta, Manuel Romero-Gomez, Jörn M. Schattenberg, Silvia Sookoian, Wendy Spearman, Norbert Stefan, Maja Thiele, Dina Tiniakos, Emmanouil Tsochatzis, Bernard Van Beers, José Willemse, Yusuf Yilmaz, and Volkan Yumuk. The authors would also like to thank the external reviewers and the EASL, EASD and EASO Governing Boards for their valuable contribution to the review process.
Figures




References
-
- Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399(10319):61–116. - PubMed
-
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–56. - PubMed
-
- European Association for the Study of the Liver . EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. - PubMed
-
- Younossi ZM, Paik JM, Stepanova M, Ong J, Alqahtani S, Henry L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J Hepatol. 2024;80(5):694–701. - PubMed
-
- Cornberg M, Tacke F, Karlsen TH, European Association for the Study of the L . Clinical Practice Guidelines of the European Association for the study of the Liver - Advancing methodology but preserving practicability. J Hepatol. 2019;70(1):5–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

