Dermatologic fungal neglected tropical diseases-Part I. Epidemiology and clinical features
- PMID: 38852743
- PMCID: PMC11970523
- DOI: 10.1016/j.jaad.2024.03.056
Dermatologic fungal neglected tropical diseases-Part I. Epidemiology and clinical features
Abstract
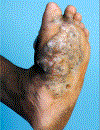
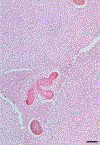
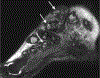
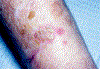
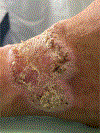
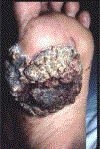
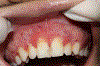
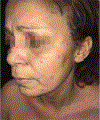
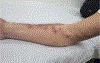
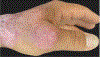
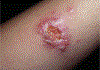
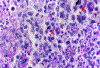
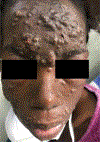
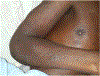
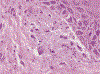
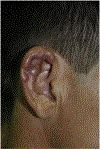
In this part 1 of a 2-part continuing medical education series, the epidemiology, clinical features, and diagnostic methods for fungal skin neglected tropical diseases (NTDs), which include eumycetoma, chromoblastomycosis, paracoccidioidomycosis, sporotrichosis, emergomycosis, talaromycosis, and lobomycosis, are reviewed. These infections, several of which are officially designated as NTDs by the World Health Organization, cause substantial morbidity and stigma worldwide and are receiving increased attention due to the potential for climate change-related geographic expansion. Domestic incidence may be increasing in the setting of global travel and immunosuppression. United States dermatologists may play a central role in early detection and initiation of appropriate treatment, leading to decreased morbidity and mortality.
Keywords: chromoblastomycosis; diagnostics; emergomycosis; endemic mycoses; epidemiology; eumycetoma; fungal infections; implantation mycoses; lobomycosis; neglected tropical diseases; paracoccidioidomycosis; sporotrichosis; systemic mycoses; talaromycosis.
Copyright © 2024 American Academy of Dermatology, Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest None disclosed.
Figures










References
-
- Gadre A, Enbiale W, Andersen LK, Coates SJ. The effects of climate change on fungal diseases with cutaneous manifestations: a report from the International Society of Dermatology Climate Change Committee. J Clim Change Health. 2022;6: 100156.
-
- Lafferty KD. The Ecology of Climate Change and Infectious Diseases. Vol. 90, Source: Ecology. 2009. - PubMed
-
- Thompson GR 3rd, Le T, Chindamporn A, Kauffman CA, Alastruey-Izquierdo A, Ampel NM, Andes DR, Armstrong-James D, Ayanlowo O, Baddley JW, Barker BM, Lopes Bezerra L, Buitrago MJ, Chamani-Tabriz L, Chan JFW, Chayakulkeeree M, Cornely OA, Cunwei C, Gangneux JP, Govender NP, Hagen F, Hedayati MT, Hohl TM, Jouvion G, Kenyon C, Kibbler CC, Klimko N, Kong DCM, Krause R, Lee Lee L, Meintjes G, Miceli MH, Rath PM, Spec A, Queiroz-Telles F, Variava E, Verweij PE, Schwartz IS, Pasqualotto AC. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis 2021. Dec;21(12):e364–e374. - PMC - PubMed
-
- Bonifaz A, Robles-Tenorio A, Tirado-Sánchez A (2022). Climate Change Impact on Chromoblastomycosis. In: Frías-De-León MG, Brunner-Mendoza C, Reyes-Montes M.d.R., Duarte-Escalante E (eds) The Impact of Climate Change on Fungal Diseases. Fungal Biology. Springer, Cham.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

