Motor assessment of X-linked dystonia parkinsonism via machine-learning-based analysis of wearable sensor data
- PMID: 38853162
- PMCID: PMC11162996
- DOI: 10.1038/s41598-024-63946-4
Motor assessment of X-linked dystonia parkinsonism via machine-learning-based analysis of wearable sensor data
Abstract
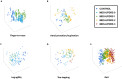
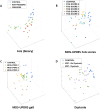
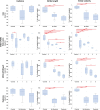
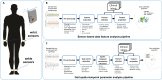
X-linked dystonia parkinsonism (XDP) is a neurogenetic combined movement disorder involving both parkinsonism and dystonia. Complex, overlapping phenotypes result in difficulties in clinical rating scale assessment. We performed wearable sensor-based analyses in XDP participants to quantitatively characterize disease phenomenology as a potential clinical trial endpoint. Wearable sensor data was collected from 10 symptomatic XDP patients and 3 healthy controls during a standardized examination. Disease severity was assessed with the Unified Parkinson's Disease Rating Scale Part 3 (MDS-UPDRS) and Burke-Fahn-Marsden dystonia scale (BFM). We collected sensor data during the performance of specific MDS-UPDRS/BFM upper- and lower-limb motor tasks, and derived data features suitable to estimate clinical scores using machine learning (ML). XDP patients were at varying stages of disease and clinical severity. ML-based algorithms estimated MDS-UPDRS scores (parkinsonism) and dystonia-specific data features with a high degree of accuracy. Gait spatio-temporal parameters had high discriminatory power in differentiating XDP patients with different MDS-UPDRS scores from controls, XDP freezing of gait, and dystonic/non-dystonic gait. These analyses suggest the feasibility of using wearable sensor data for deriving reliable clinical score estimates associated with both parkinsonian and dystonic features in a complex, combined movement disorder and the utility of motion sensors in quantifying clinical examination.
Keywords: Digital health; Dystonia; Dystonia parkinsonism; Machine Learning; Parkinsonism; Wearable sensors.
© 2024. The Author(s).
Conflict of interest statement
Dr. Parisi reports no relevant disclosures. Dr. Corniani reports no relevant disclosures. Dr. Bonato has received grant support from the American Heart Association, the Department of Defense, the Michael J Fox Foundation, the National Institutes of Health (NIH), the National Science Foundation (NSF), and the Peabody Foundation including sub-awards on NIH and NSF SBIR grants from Barrett Technology (Newton MA), BioSensics (Watertown MA) and Veristride (Salt Lake City UT). He has also received grant support from Emerge Diagnostics (Carlsbad CA), MC10 (Lexington MA), Mitsui Chemicals (Tokyo Japan), Pfizer (New York City NY), Shimmer Research (Dublin Ireland), and SynPhNe (Singapore). He has served on the Advisory Board of SwanBio (Boston MA). Dr. Bonato serves in an advisory uncompensated role the Michael J Fox Foundation, the NIH-funded New England Pediatric Device Consortium, and the Walking Tall-PD clinical trial carried out by Neuroscience Research Australia. He also serves in an uncompensated role on the Scientific Advisory Boards of ABLE Human Motion (Barcelona, Spain), FormSense (San Diego CA, USA), Hocoma AG (Zurich, Switzerland), and Trexo (Toronto, Canada). Dr. Acuna reports no relevant disclosures. Dr. Go reports no relevant disclosures. Dr. Sharma receives support from Wiley Publishing for her role as editor-in-chief of Brain and Behavior. has received grant support from the Department of Defense and the National Institutes of Health (NIH). Dr. Stephen has received financial support from Encora Therapeutics, SwanBio Therapeutics, Sanofi-Genzyme, Biogen and Biohaven for the conduct of clinical trials. He has received honoraria from the Movement Disorders Society, American Academy of Neurology and from Oakstone CME. Dr. Stephen’s institution has received research funding from Sanofi-Genzyme for a study of video oculography in late-onset GM2 gangliosidosis.
Figures

References
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

