Elevated nuclear TDP-43 induces constitutive exon skipping
- PMID: 38853250
- PMCID: PMC11163724
- DOI: 10.1186/s13024-024-00732-w
Elevated nuclear TDP-43 induces constitutive exon skipping
Abstract
Background: Cytoplasmic inclusions and loss of nuclear TDP-43 are key pathological features found in several neurodegenerative disorders, suggesting both gain- and loss-of-function mechanisms of disease. To study gain-of-function, TDP-43 overexpression has been used to generate in vitro and in vivo model systems.
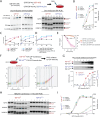
Methods: We analyzed RNA-seq datasets from mouse and human neurons overexpressing TDP-43 to explore species specific splicing patterns. We explored the dynamics between TDP-43 levels and exon repression in vitro. Furthermore we analyzed human brain samples and publicly available RNA datasets to explore the relationship between exon repression and disease.
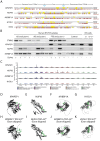
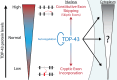
Results: Our study shows that excessive levels of nuclear TDP-43 protein lead to constitutive exon skipping that is largely species-specific. Furthermore, while aberrant exon skipping is detected in some human brains, it is not correlated with disease, unlike the incorporation of cryptic exons that occurs after loss of TDP-43.
Conclusions: Our findings emphasize the need for caution in interpreting TDP-43 overexpression data and stress the importance of controlling for exon skipping when generating models of TDP-43 proteinopathy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Update of
-
Elevated nuclear TDP-43 induces constitutive exon skipping.bioRxiv [Preprint]. 2023 May 12:2023.05.11.540291. doi: 10.1101/2023.05.11.540291. bioRxiv. 2023. Update in: Mol Neurodegener. 2024 Jun 9;19(1):45. doi: 10.1186/s13024-024-00732-w. PMID: 37215013 Free PMC article. Updated. Preprint.
Similar articles
-
Long-read RNA sequencing unveils a novel cryptic exon in MNAT1 along with its full-length transcript structure in TDP-43 proteinopathy.Commun Biol. 2025 Jul 16;8(1):1056. doi: 10.1038/s42003-025-08463-4. Commun Biol. 2025. PMID: 40670663 Free PMC article.
-
Elevated nuclear TDP-43 induces constitutive exon skipping.bioRxiv [Preprint]. 2023 May 12:2023.05.11.540291. doi: 10.1101/2023.05.11.540291. bioRxiv. 2023. Update in: Mol Neurodegener. 2024 Jun 9;19(1):45. doi: 10.1186/s13024-024-00732-w. PMID: 37215013 Free PMC article. Updated. Preprint.
-
TDP-43 Cryptic RNAs in Perry Syndrome: Differences across Brain Regions and TDP-43 Proteinopathies.Mov Disord. 2025 Apr;40(4):662-671. doi: 10.1002/mds.30104. Epub 2025 Jan 9. Mov Disord. 2025. PMID: 39788898 Free PMC article.
-
The Role of TDP-43 in SARS-CoV-2-Related Neurodegenerative Changes.Viruses. 2025 May 19;17(5):724. doi: 10.3390/v17050724. Viruses. 2025. PMID: 40431734 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
A robust evaluation of TDP-43, poly GP, cellular pathology and behavior in a AAV-C9ORF72 (G4C2)66 mouse model.bioRxiv [Preprint]. 2024 Aug 27:2024.08.27.607409. doi: 10.1101/2024.08.27.607409. bioRxiv. 2024. Update in: Acta Neuropathol Commun. 2024 Dec 26;12(1):203. doi: 10.1186/s40478-024-01911-y. PMID: 39253499 Free PMC article. Updated. Preprint.
-
Critical impact of lysine 136 in TDP-43 phase separation, compartmentalization, and aggregation in living vertebrates.iScience. 2025 May 27;28(7):112761. doi: 10.1016/j.isci.2025.112761. eCollection 2025 Jul 18. iScience. 2025. PMID: 40585370 Free PMC article.
-
Viral-mediated knockdown of Atxn2 attenuates TDP-43 pathology and muscle dysfunction in the PFN1C71G ALS mouse model.Acta Neuropathol Commun. 2025 May 24;13(1):116. doi: 10.1186/s40478-025-02005-z. Acta Neuropathol Commun. 2025. PMID: 40413526 Free PMC article.
-
Alternative 3' UTR polyadenylation is disrupted in the rNLS8 mouse model of ALS/FTLD.Mol Brain. 2025 Jan 14;18(1):1. doi: 10.1186/s13041-025-01174-1. Mol Brain. 2025. PMID: 39810199 Free PMC article.
-
Long-read RNA sequencing unveils a novel cryptic exon in MNAT1 along with its full-length transcript structure in TDP-43 proteinopathy.Commun Biol. 2025 Jul 16;8(1):1056. doi: 10.1038/s42003-025-08463-4. Commun Biol. 2025. PMID: 40670663 Free PMC article.
References
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM-Y. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. - DOI - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Bioph Res Co. 2006;351:602–11. doi: 10.1016/j.bbrc.2006.10.093. - DOI - PubMed
-
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, Coyle-Gilchrist ITS, Chui HC, Fardo DW, Flanagan ME, Halliday G, Hokkanen SRK, Hunter S, Jicha GA, Katsumata Y, Kawas CH, Keene CD, Kovacs GG, Kukull WA, Levey AI, Makkinejad N, Montine TJ, Murayama S, Murray ME, Nag S, Rissman RA, Seeley WW, Sperling RA, Schneider CLW. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142:1503-1527. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

