Soluble Fibrinogen-Like Protein 2 Downregulation and Th17/Treg Imbalance in a Taurocholate-Induced Murine Experimental Model of Severe Acute Pancreatitis
- PMID: 38853390
- PMCID: PMC11211668
- DOI: 10.1002/jcla.25076
Soluble Fibrinogen-Like Protein 2 Downregulation and Th17/Treg Imbalance in a Taurocholate-Induced Murine Experimental Model of Severe Acute Pancreatitis
Abstract
Background: Severe acute pancreatitis (SAP) is associated with tremendous systemic inflammation, T-helper 17 (Th17) cells, and regulatory T (Treg) cells play an essential role in the inflammatory responses. Meanwhile, soluble fibrinogen-like protein 2 (Sfgl2) is a critical immunosuppressive effector cytokine of Treg cells and modulates immune responses. However, the impact of SAP induction on Sfgl2 expression and the role of Sfgl2 in immunomodulation under SAP conditions are largely unknown.
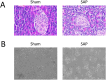
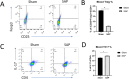
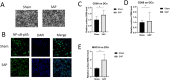
Methods: A taurocholate-induced mouse SAP model was established. The ratios of CD4+CD25+Foxp3+ Treg cells or CD4+IL-17+ Th17 cells in blood and pancreatic tissues as well as surface expression of CD80, CD86, and major histocompatibility complex class II (MHC-II) were determined by flow cytometry. Gene mRNA expression was determined by qPCR. Serum amylase and soluble factors were quantitated by commercial kits. Bone marrow-derived dendritic cells (DCs) were generated, and NF-κB/p65 translocation was measured by immunofluorescence staining.
Results: SAP induction in mice decreased the Th17/Treg ratio in the pancreatic tissue and increased the Th17/Treg ratio in the peripheral blood. In addition, SAP was associated with a reduced level of Sfgl2 in the pancreatic tissue and blood: higher levels of serum IL-17, IL-2, IFN-α, and TNF-α, and lower levels of serum IL-4 and IL-10. Furthermore, the SAP-induced reduction in Sfgl2 expression was accompanied by dysregulated maturation of bone marrow-derived DCs.
Conclusions: SAP causes reduced Sfgl2 expression and Th17/Treg imbalance, thus providing critical insights for the development of Sfgl2- and Th17/Treg balance-targeted immunotherapies for patients with SAP.
Keywords: Th17/Treg imbalance; T‐helper 17 cell; immunomodulation; inflammation; regulatory T cell; severe acute pancreatitis; soluble fibrinogen–like protein 2.
© 2024 The Author(s). Journal of Clinical Laboratory Analysis published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Correlation of fibrinogen-like protein 2 with progression of acute pancreatitis in rats.World J Gastroenterol. 2013 Apr 28;19(16):2492-500. doi: 10.3748/wjg.v19.i16.2492. World J Gastroenterol. 2013. PMID: 23674850 Free PMC article.
-
Soluble fibrinogen-like protein 2 promotes the growth of hepatocellular carcinoma via attenuating dendritic cell-mediated cytotoxic T cell activity.J Exp Clin Cancer Res. 2019 Aug 13;38(1):351. doi: 10.1186/s13046-019-1326-5. J Exp Clin Cancer Res. 2019. PMID: 31409352 Free PMC article.
-
Soluble Fgl2 restricts autoimmune hepatitis progression via suppressing Tc17 and conventional CD8+ T cell function.J Gene Med. 2018 Jul;20(7-8):e3023. doi: 10.1002/jgm.3023. Epub 2018 Jun 19. J Gene Med. 2018. PMID: 29756667
-
Soluble fibrinogen like protein 2 (sFGL2), the novel effector molecule for immunoregulation.Oncotarget. 2017 Jan 10;8(2):3711-3723. doi: 10.18632/oncotarget.12533. Oncotarget. 2017. PMID: 27732962 Free PMC article. Review.
-
Th17/Treg Cell Imbalance May Contribute to Spontaneous Preterm Labor.J Immunol Res. 2025 May 22;2025:8405365. doi: 10.1155/jimr/8405365. eCollection 2025. J Immunol Res. 2025. PMID: 40443571 Free PMC article. Review.
Cited by
-
The role of ischaemia-modified albumin in the prognosis of acute pancreatitis and its correlation with the NF-κB-mediated inflammatory response.J Int Med Res. 2024 Oct;52(10):3000605241287163. doi: 10.1177/03000605241287163. J Int Med Res. 2024. PMID: 39474645 Free PMC article.
References
-
- Lankisch P. G., Apte M., and Banks P. A., “Acute Pancreatitis,” Lancet 386 (2015): 85–96. - PubMed
-
- van Baal M. C., van Santvoort H. C., Bollen T. L., et al., “Systematic Review of Percutaneous Catheter Drainage as Primary Treatment for Necrotizing Pancreatitis,” British Journal of Surgery 98 (2011): 18–27. - PubMed
-
- Zerem E., Imamovic G., Susic A., and Haracic B., “Step‐Up Approach to Infected Necrotising Pancreatitis: A 20‐Year Experience of Percutaneous Drainage in a Single Centre,” Digestive and Liver Disease 43 (2011): 478–483. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

