Discovery and biological profile of pyridachlometyl
- PMID: 38853401
- PMCID: PMC11981980
- DOI: 10.1002/ps.8239
Discovery and biological profile of pyridachlometyl
Abstract
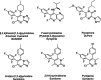
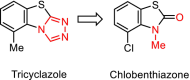
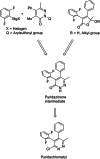
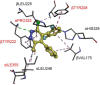
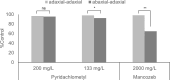
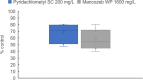
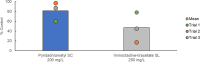
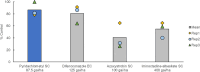
Pyridachlometyl is a novel tubulin dynamics modulator fungicide developed by Sumitomo as a new agent designed to tackle fungicide resistance. Pyridachlometyl is being developed as a first-in-class molecule with an anti-tubulin mode of action, the chemical structure of which is characterized by a unique tetrasubstituted pyridazine ring. The first commercial product 'Fuseki flowable' received initial registration in 2023 in Japan. The concepts of the discovery project, optimization of chemical structures, and biological profiles are reviewed herein. © 2024 The Author(s). Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: bioisostere; fungicide resistance; pyridachlometyl; pyridazine; tubulin.
© 2024 The Author(s). Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Figures
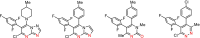


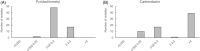

Similar articles
-
Discovery of pyridachlometyl: A new class of pyridazine fungicides.Bioorg Med Chem. 2023 Jun 6;88-89:117332. doi: 10.1016/j.bmc.2023.117332. Epub 2023 May 16. Bioorg Med Chem. 2023. PMID: 37210791
-
Pyridachlometyl has a novel anti-tubulin mode of action which could be useful in anti-resistance management.Pest Manag Sci. 2020 Apr;76(4):1393-1401. doi: 10.1002/ps.5652. Epub 2019 Nov 12. Pest Manag Sci. 2020. PMID: 31622533 Free PMC article.
-
Synthesis and fungicidal activity of tubulin polymerisation promoters. Part 2: pyridazines.Bioorg Med Chem. 2012 May 1;20(9):2803-10. doi: 10.1016/j.bmc.2012.03.035. Epub 2012 Mar 24. Bioorg Med Chem. 2012. PMID: 22494843
-
Discovery, research and development of axalion® active insecticide: dimpropyridaz†.Pest Manag Sci. 2025 May;81(5):2529-2534. doi: 10.1002/ps.8385. Epub 2024 Sep 25. Pest Manag Sci. 2025. PMID: 39320022 Free PMC article. Review.
-
Isoflucypram, the first representative of a new succinate dehydrogenase inhibitor fungicide subclass: Its chemical discovery and unusual binding mode.Pest Manag Sci. 2020 Oct;76(10):3340-3347. doi: 10.1002/ps.5951. Epub 2020 Jul 2. Pest Manag Sci. 2020. PMID: 32506626 Free PMC article. Review.
References
-
- Stukenbrock E and Gurr S, Address the growing urgency of fungal disease in crops. Nature 617:31–34 (2023). - PubMed
-
- Hollomon DH, Fungicide resistance: 40 years on and still a major problem, in Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management, ed. by Hollomon DH and Ishii H. Springer Tokyo, Tokyo, Japan, pp. 3–12 (2015).
-
- Young DH, Anti‐tubulin agents, in Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management, ed. by Hollomon DH and Ishii H. Springer Tokyo, Tokyo, Japan, pp. 93–104 (2015).
-
- Kato RB, Jaiswal AK, Tiwari S, Barh D, Azevedo V and Góes‐Neto A, Chapter 12 – pan‐genomics of fungi and its applications, in Pan‐Genomics: Applications, Challenges, and Future Prospects, ed. by Barh D, Soares S and Tiwari Sand Azevedo V. Academic Press, Cambridge, MA, USA, pp. 251–260 (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

