Metabolic Reprogramming in Thyroid Cancer
- PMID: 38853437
- PMCID: PMC11220218
- DOI: 10.3803/EnM.2023.1802
Metabolic Reprogramming in Thyroid Cancer
Abstract
Thyroid cancer is a common endocrine malignancy with increasing incidence globally. Although most cases can be treated effectively, some cases are more aggressive and have a higher risk of mortality. Inhibiting RET and BRAF kinases has emerged as a potential therapeutic strategy for the treatment of thyroid cancer, particularly in cases of advanced or aggressive disease. However, the development of resistance mechanisms may limit the efficacy of these kinase inhibitors. Therefore, developing precise strategies to target thyroid cancer cell metabolism and overcome resistance is a critical area of research for advancing thyroid cancer treatment. In the field of cancer therapeutics, researchers have explored combinatorial strategies involving dual metabolic inhibition and metabolic inhibitors in combination with targeted therapy, chemotherapy, and immunotherapy to overcome the challenge of metabolic plasticity. This review highlights the need for new therapeutic approaches for thyroid cancer and discusses promising metabolic inhibitors targeting thyroid cancer. It also discusses the challenges posed by metabolic plasticity in the development of effective strategies for targeting cancer cell metabolism and explores the potential advantages of combined metabolic targeting.
Keywords: Drug resistance, neoplasm; Immunotherapy; Metabolic networks and pathways; Thyroid neoplasms; Tyrosine kinase inhibitors.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
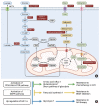
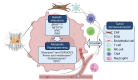
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Sanchez-Ares M, Cameselle-Garcia S, Abdulkader-Nallib I, Rodriguez-Carnero G, Beiras-Sarasquete C, Punal-Rodriguez JA, et al. Susceptibility genes and chromosomal regions associated with non-syndromic familial non-medullary thyroid carcinoma: some pathogenetic and diagnostic keys. Front Endocrinol (Lausanne) 2022;13:829103. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

